当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reduction and Scavenging of Chemically Reactive Drug Metabolites by NAD(P)H:Quinone Oxidoreductase 1 and NRH:Quinone Oxidoreductase 2 and Variability in Hepatic Concentrations.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2018-01-11 , DOI: 10.1021/acs.chemrestox.7b00289 Shalenie P den Braver-Sewradj 1 , Michiel W den Braver 1 , Robin M Toorneman 1 , Stephanie van Leeuwen 1 , Yongjie Zhang 1 , Stefan J Dekker 1 , Nico P E Vermeulen 1 , Jan N M Commandeur 1 , J Chris Vos 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2018-01-11 , DOI: 10.1021/acs.chemrestox.7b00289 Shalenie P den Braver-Sewradj 1 , Michiel W den Braver 1 , Robin M Toorneman 1 , Stephanie van Leeuwen 1 , Yongjie Zhang 1 , Stefan J Dekker 1 , Nico P E Vermeulen 1 , Jan N M Commandeur 1 , J Chris Vos 1
Affiliation
|
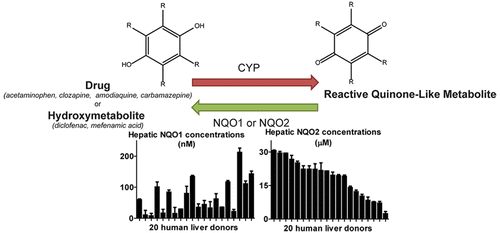
Detoxicating enzymes NAD(P)H:quinone oxidoreductase 1 (NQO1) and NRH:quinone oxidoreductase 2 (NQO2) catalyze the two-electron reduction of quinone-like compounds. The protective role of the polymorphic NQO1 and NQO2 enzymes is especially of interest in the liver as the major site of drug bioactivation to chemically reactive drug metabolites. In the current study, we quantified the concentrations of NQO1 and NQO2 in 20 human liver donors and NQO1 and NQO2 activities with quinone-like drug metabolites. Hepatic NQO1 concentrations ranged from 8 to 213 nM. Using recombinant NQO1, we showed that low nM concentrations of NQO1 are sufficient to reduce synthetic amodiaquine and carbamazepine quinone-like metabolites in vitro. Hepatic NQO2 concentrations ranged from 2 to 31 μM. NQO2 catalyzed the reduction of quinone-like metabolites derived from acetaminophen, clozapine, 4'-hydroxydiclofenac, mefenamic acid, amodiaquine, and carbamazepine. The reduction of the clozapine nitrenium ion supports association studies showing that NQO2 is a genetic risk factor for clozapine-induced agranulocytosis. The 5-hydroxydiclofenac quinone imine, which was previously shown to be reduced by NQO1, was not reduced by NQO2. Tacrine was identified as a potent NQO2 inhibitor and was applied to further confirm the catalytic activity of NQO2 in these assays. While the in vivo relevance of NQO2-catalyzed reduction of quinone-like metabolites remains to be established by identification of the physiologically relevant co-substrates, our results suggest an additional protective role of the NQO2 protein by non-enzymatic scavenging of quinone-like metabolites. Hepatic NQO1 activity in detoxication of quinone-like metabolites becomes especially important when other detoxication pathways are exhausted and NQO1 levels are induced.
中文翻译:

NAD(P)H:醌醌氧化还原酶1和NRH:醌醌氧化还原酶2对化学反应性药物代谢产物的还原和清除作用以及肝中浓度的变异性。
脱毒酶NAD(P)H:醌氧化还原酶1(NQO1)和NRH:醌氧化还原酶2(NQO2)催化类醌化合物的双电子还原。在肝脏中,多态性NQO1和NQO2酶的保护作用特别引起人们的兴趣,因为它是药物对化学反应性药物代谢物进行生物活化的主要部位。在当前的研究中,我们量化了20种人类肝脏供体中NQO1和NQO2的浓度以及类似醌类药物代谢产物的NQO1和NQO2活性。肝NQO1浓度范围为8到213 nM。使用重组NQO1,我们显示低nM的NQO1浓度足以在体外减少合成的阿莫地喹和卡马西平醌类代谢物。肝脏NQO2浓度范围为2到31μM。NQO2催化了对乙酰氨基酚,氯氮平,4'-羟基双氯芬酸,芬那那酸,阿莫地喹和卡马西平衍生的醌类代谢物的还原。氯氮平氮离子的减少支持相关研究,表明NQO2是氯氮平诱导的粒细胞缺乏症的遗传危险因素。先前显示可被NQO1还原的5-羟基双氯芬酸醌亚胺不会被NQO2还原。他克林被鉴定为有效的NQO2抑制剂,并用于进一步证实NQO2在这些测定中的催化活性。虽然在体内与NQO2催化的类似醌的代谢物还原的相关性仍有待通过生理上相关的共底物的鉴定来确定,我们的结果表明,通过非酶清除醌类代谢物,NQO2蛋白具有额外的保护作用。当耗尽其他脱毒途径并诱导NQO1水平时,肝类NQO1在使醌类代谢物解毒中的活性变得尤为重要。
更新日期:2018-01-11
中文翻译:

NAD(P)H:醌醌氧化还原酶1和NRH:醌醌氧化还原酶2对化学反应性药物代谢产物的还原和清除作用以及肝中浓度的变异性。
脱毒酶NAD(P)H:醌氧化还原酶1(NQO1)和NRH:醌氧化还原酶2(NQO2)催化类醌化合物的双电子还原。在肝脏中,多态性NQO1和NQO2酶的保护作用特别引起人们的兴趣,因为它是药物对化学反应性药物代谢物进行生物活化的主要部位。在当前的研究中,我们量化了20种人类肝脏供体中NQO1和NQO2的浓度以及类似醌类药物代谢产物的NQO1和NQO2活性。肝NQO1浓度范围为8到213 nM。使用重组NQO1,我们显示低nM的NQO1浓度足以在体外减少合成的阿莫地喹和卡马西平醌类代谢物。肝脏NQO2浓度范围为2到31μM。NQO2催化了对乙酰氨基酚,氯氮平,4'-羟基双氯芬酸,芬那那酸,阿莫地喹和卡马西平衍生的醌类代谢物的还原。氯氮平氮离子的减少支持相关研究,表明NQO2是氯氮平诱导的粒细胞缺乏症的遗传危险因素。先前显示可被NQO1还原的5-羟基双氯芬酸醌亚胺不会被NQO2还原。他克林被鉴定为有效的NQO2抑制剂,并用于进一步证实NQO2在这些测定中的催化活性。虽然在体内与NQO2催化的类似醌的代谢物还原的相关性仍有待通过生理上相关的共底物的鉴定来确定,我们的结果表明,通过非酶清除醌类代谢物,NQO2蛋白具有额外的保护作用。当耗尽其他脱毒途径并诱导NQO1水平时,肝类NQO1在使醌类代谢物解毒中的活性变得尤为重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号