当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen Evolution Reaction in Alkaline Media: Alpha- or Beta-Nickel Hydroxide on the Surface of Platinum?
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acsenergylett.7b01103 Xiaowen Yu 1 , Jun Zhao 2 , Li-Rong Zheng 3 , Yue Tong 1 , Miao Zhang 1 , Guochuang Xu 1 , Chun Li 1 , Jing Ma 2 , Gaoquan Shi 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acsenergylett.7b01103 Xiaowen Yu 1 , Jun Zhao 2 , Li-Rong Zheng 3 , Yue Tong 1 , Miao Zhang 1 , Guochuang Xu 1 , Chun Li 1 , Jing Ma 2 , Gaoquan Shi 1
Affiliation
|
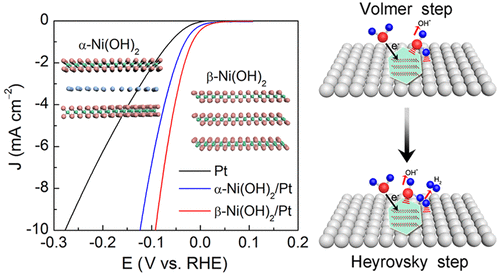
Reducing the energy consumption of a hydrogen evolution reaction (HER) at a platinum (Pt) electrode is important for the hydrogen economy. Herein, we report the loading of alpha- or beta-nickel hydroxide (α- or β-Ni(OH)2) nanostructures on the surface of a Pt electrode to improve its catalytic activity and stability for HER in alkaline electrolytes. Both experimental and theoretical studies reveal that β-Ni(OH)2 is a better co-catalyst of Pt than α-Ni(OH)2 for promoting the HER, attributed to the higher water dissociation ability of β-Ni(OH)2, as well as the stronger interactions between β-Ni(OH)2 and the Pt electrode. Particularly, the overpotential of the HER in 0.1 M KOH at 10 mA cm–2 is decreased from 278 mV at the Pt electrode to 92 mV at the β-Ni(OH)2/Pt electrode, and the Tafel slope decreased from 62 to 42 mV dec–1, correspondingly. The performance of the β-Ni(OH)2/Pt catalytic electrode surpasses that of most of the previously reported electrodes for the same purpose.
中文翻译:

碱性介质中的氢释放反应:铂表面上的α-或β-镍氢氧化物?
减少铂(Pt)电极上的析氢反应(HER)的能量消耗对于节约氢非常重要。在此,我们报告了Pt电极表面上的α-或β-镍氢氧化物(α-或β-Ni(OH)2)纳米结构的负载,以提高其在碱性电解质中对HER的催化活性和稳定性。实验和理论研究均表明,β-Ni(OH)2比α-Ni(OH)2更好的Pt助催化剂促进了HER,这归因于β-Ni(OH)2的更高的水分解能力。以及β-Ni(OH)2和Pt电极之间更强的相互作用。特别是,在10 mA cm –2的条件下,HER在0.1 M KOH中的过电势从Pt电极上的278 mV降低到β-Ni(OH)2 / Pt电极上的92 mV,Tafel斜率相应地从62 mV dec –1降低。为了相同的目的,β-Ni(OH)2 / Pt催化电极的性能超过了大多数先前报道的电极的性能。
更新日期:2017-12-27
中文翻译:

碱性介质中的氢释放反应:铂表面上的α-或β-镍氢氧化物?
减少铂(Pt)电极上的析氢反应(HER)的能量消耗对于节约氢非常重要。在此,我们报告了Pt电极表面上的α-或β-镍氢氧化物(α-或β-Ni(OH)2)纳米结构的负载,以提高其在碱性电解质中对HER的催化活性和稳定性。实验和理论研究均表明,β-Ni(OH)2比α-Ni(OH)2更好的Pt助催化剂促进了HER,这归因于β-Ni(OH)2的更高的水分解能力。以及β-Ni(OH)2和Pt电极之间更强的相互作用。特别是,在10 mA cm –2的条件下,HER在0.1 M KOH中的过电势从Pt电极上的278 mV降低到β-Ni(OH)2 / Pt电极上的92 mV,Tafel斜率相应地从62 mV dec –1降低。为了相同的目的,β-Ni(OH)2 / Pt催化电极的性能超过了大多数先前报道的电极的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号