当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Imide–Orthoborate Dual-Salt Mixtures in Organic Carbonate Electrolytes on the Stability of Lithium Metal Batteries
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acsami.7b15117 Xing Li 1, 2 , Jianming Zheng 1 , Mark H. Engelhard 3 , Donghai Mei 4 , Qiuyan Li 1 , Shuhong Jiao 1 , Ning Liu 4, 5 , Wengao Zhao 1, 6 , Ji-Guang Zhang 1 , Wu Xu 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acsami.7b15117 Xing Li 1, 2 , Jianming Zheng 1 , Mark H. Engelhard 3 , Donghai Mei 4 , Qiuyan Li 1 , Shuhong Jiao 1 , Ning Liu 4, 5 , Wengao Zhao 1, 6 , Ji-Guang Zhang 1 , Wu Xu 1
Affiliation
|
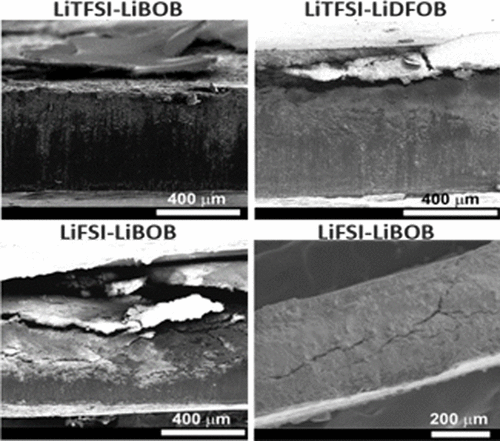
The effects of lithium imide and lithium orthoborate dual-salt electrolytes of different salt chemistries in carbonate solvents on the cycling stability of lithium (Li) metal batteries are systematically and comparatively investigated. Two imide salts (LiTFSI and LiFSI) and two orthoborate salts (LiBOB and LiDFOB) are chosen for this study and compared with the conventional LiPF6 salt. Density functional theory calculations indicate that the chemical and electrochemical stabilities rank in the following order: LiTFSI-LiBOB > LiTFSI-LiDFOB > LiFSI-LiDFOB > LiFSI-LiBOB. The experimental cycling stability of the Li metal batteries with the electrolytes ranks in the following order: LiTFSI-LiBOB > LiTFSI-LiDFOB > LiFSI-LiDFOB > LiPF6 > LiFSI-LiBOB, which is in well accordance with the calculation results. The LiTFSI-LiBOB can effectively protect the Al substrate and form a more robust surface film on Li metal anode, while the LiFSI-LiBOB results in serious corrosion to the stainless steel cell case and a thicker and looser surface film on Li anode. The key findings of this work emphasize that the salt chemistry is critically important for enhancing the interfacial stability of Li metal anode and should be carefully manipulated in the development of high-performance Li metal batteries.
中文翻译:

有机碳酸盐电解质中酰亚胺-原硼酸酯双盐混合物对锂金属电池稳定性的影响
系统地和比较地研究了碳酸锂溶剂中不同盐化学类型的酰亚胺锂和原硼酸锂双盐电解质对锂(Li)金属电池循环稳定性的影响。本研究选择了两种酰亚胺盐(LiTFSI和LiFSI)和两种原硼酸盐(LiBOB和LiDFOB),并与常规LiPF 6盐进行了比较。密度泛函理论计算表明,化学和电化学稳定性按以下顺序排列:LiTFSI-LiBOB> LiTFSI-LiDFOB> LiFSI-LiDFOB> LiFSI-LiBOB。具有电解质的锂金属电池的实验循环稳定性按以下顺序排列:LiTFSI-LiBOB> LiTFSI-LiDFOB> LiFSI-LiDFOB> LiPF 6> LiFSI-LiBOB,这与计算结果完全一致。LiTFSI-LiBOB可以有效保护Al基材并在Li金属阳极上形成更坚固的表面膜,而LiFSI-LiBOB则严重腐蚀不锈钢电池外壳,并在Li阳极上形成更厚更疏的表面膜。这项工作的关键发现强调,盐化学对于增强锂金属阳极的界面稳定性至关重要,在开发高性能锂金属电池时应谨慎操作。
更新日期:2018-01-12
中文翻译:

有机碳酸盐电解质中酰亚胺-原硼酸酯双盐混合物对锂金属电池稳定性的影响
系统地和比较地研究了碳酸锂溶剂中不同盐化学类型的酰亚胺锂和原硼酸锂双盐电解质对锂(Li)金属电池循环稳定性的影响。本研究选择了两种酰亚胺盐(LiTFSI和LiFSI)和两种原硼酸盐(LiBOB和LiDFOB),并与常规LiPF 6盐进行了比较。密度泛函理论计算表明,化学和电化学稳定性按以下顺序排列:LiTFSI-LiBOB> LiTFSI-LiDFOB> LiFSI-LiDFOB> LiFSI-LiBOB。具有电解质的锂金属电池的实验循环稳定性按以下顺序排列:LiTFSI-LiBOB> LiTFSI-LiDFOB> LiFSI-LiDFOB> LiPF 6> LiFSI-LiBOB,这与计算结果完全一致。LiTFSI-LiBOB可以有效保护Al基材并在Li金属阳极上形成更坚固的表面膜,而LiFSI-LiBOB则严重腐蚀不锈钢电池外壳,并在Li阳极上形成更厚更疏的表面膜。这项工作的关键发现强调,盐化学对于增强锂金属阳极的界面稳定性至关重要,在开发高性能锂金属电池时应谨慎操作。











































 京公网安备 11010802027423号
京公网安备 11010802027423号