当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Concise Synthesis of Natural Phenylphenalenone Phytoalexins and a Regioisomer
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00709 Ming-Zhong Wang 1, 2 , Chuen-Fai Ku 2 , Tong-Xu Si 1 , Siu-Wai Tsang 2 , Xiao-Meng Lv 1 , Xiao-Wan Li 1 , Zheng-Ming Li 3 , Hong-Jie Zhang 2 , Albert S. C. Chan 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00709 Ming-Zhong Wang 1, 2 , Chuen-Fai Ku 2 , Tong-Xu Si 1 , Siu-Wai Tsang 2 , Xiao-Meng Lv 1 , Xiao-Wan Li 1 , Zheng-Ming Li 3 , Hong-Jie Zhang 2 , Albert S. C. Chan 1
Affiliation
|
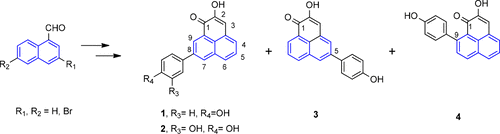
Concise total syntheses of the natural phytoalexins 2-hydroxy-8-(4-hydroxyphenyl)phenalen-1-one (1), 2-hydroxy-8-(3,4-dihydroxyphenyl)phenalen-1-one (2), and hydroxyanigorufone (4), together with regioisomer 3 are accomplished in 11 or 12 steps. The synthetic strategy features a Friedel–Crafts acylation to construct the 1H-phenalen-1-one tricyclic core followed by a Suzuki cross-coupling to obtain the target compounds.
中文翻译:

天然苯甲酚酮植物抗毒素和区域异构体的简捷合成
天然植物抗毒素2-羟基-8-(4-羟基苯基)苯并-1-酮(1),2-羟基-8-(3,4-二羟基苯基)苯并-1-酮(2)的简明合成方法羟基苯并呋喃酮(4)与区域异构体3一起以11或12个步骤完成。合成策略的特征在于,Friedel-Crafts酰化反应可构建1 H -phenalen-1-one三环核,然后进行Suzuki交叉偶联以获得目标化合物。
更新日期:2017-12-27
中文翻译:

天然苯甲酚酮植物抗毒素和区域异构体的简捷合成
天然植物抗毒素2-羟基-8-(4-羟基苯基)苯并-1-酮(1),2-羟基-8-(3,4-二羟基苯基)苯并-1-酮(2)的简明合成方法羟基苯并呋喃酮(4)与区域异构体3一起以11或12个步骤完成。合成策略的特征在于,Friedel-Crafts酰化反应可构建1 H -phenalen-1-one三环核,然后进行Suzuki交叉偶联以获得目标化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号