当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determining Linear Free Energy Relationships in Peptide Fragmentation Using Derivatization and Targeted Mass Spectrometry
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.analchem.7b02191 Yuanyuan Shen 1 , Reza Nemati 1 , Lei Wang 1 , Xudong Yao 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.analchem.7b02191 Yuanyuan Shen 1 , Reza Nemati 1 , Lei Wang 1 , Xudong Yao 1
Affiliation
|
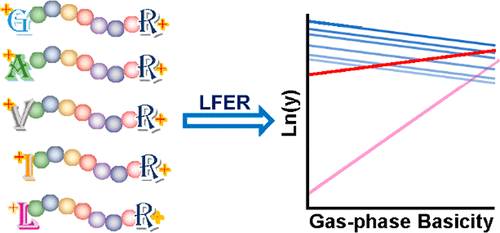
The sequence complexity of a proteome is utilized with rational chemical derivatization to establish the linear free energy relationship (LFER) in order to investigate the collision-induced dissociation (CID) of peptides. The derivatization produces groups of peptides that have varying designer residues of aliphatic amino acids at the N-terminus but stay invariable for the rest of sequences, which are naturally occurring and uncontrolled. Collisional fragmentation of the derivatized peptides is advantageously monitored by liquid-chromatography multiple-reaction-monitoring mass spectrometry. Systematically tuning the gas-phase basicity of the N-termini of peptides establishes LFERs that report the structural similarities and differences in CID of all the backbone amides of doubly protonated tryptic peptides. For the cleavage of an internal or C-terminal amide, the peptide N-terminus mainly affects the mobility of the N-terminal proton instead of directly participating in the amide cleavage. In contrast, the terminal residue plays more pronounced roles in the cleavage of the first and second amide bonds. LFERs for the competition between the symmetric and asymmetric cleavage of the second amide bond support the protonated oxazolone structure for N-terminal fragments. This competition is affected locally by the chemistry of the first three residues and remotely by charge repulsion between the two protonation sites.
中文翻译:

使用衍生化和靶向质谱法确定肽片段化中的线性自由能关系
蛋白质组的序列复杂性与合理的化学衍生作用一起使用,以建立线性自由能关系(LFER),以研究肽的碰撞诱导解离(CID)。衍生化产生的肽组在N端具有不同的脂族氨基酸设计者残基,但对于自然存在且不受控制的其余序列保持不变。通过液相色谱多反应监测质谱法有利地监测衍生肽的碰撞碎裂。系统地调节肽N末端的气相碱性会建立LFER,这些LFER报告双质子化胰蛋白酶肽的所有主链酰胺的CID的结构相似性和差异。对于内部或C端酰胺的裂解,肽N端主要影响N端质子的迁移,而不是直接参与酰胺裂解。相反,末端残基在第一和第二酰胺键的裂解中起更明显的作用。用于第二酰胺键的对称和不对称裂解之间竞争的LFER支持N端片段的质子化恶唑酮结构。竞争受前三个残基的化学作用局部影响,而两个质子化位点之间的电荷排斥作用则受到远程影响。末端残基在第一和第二酰胺键的裂解中起更明显的作用。用于第二酰胺键的对称和不对称裂解之间竞争的LFER支持N端片段的质子化恶唑酮结构。竞争受前三个残基的化学作用局部影响,而两个质子化位点之间的电荷排斥作用则受到远程影响。末端残基在第一和第二酰胺键的裂解中起更明显的作用。用于第二酰胺键的对称和不对称裂解之间竞争的LFER支持N端片段的质子化恶唑酮结构。竞争受前三个残基的化学作用局部影响,而两个质子化位点之间的电荷排斥作用则受到远程影响。
更新日期:2018-01-12
中文翻译:

使用衍生化和靶向质谱法确定肽片段化中的线性自由能关系
蛋白质组的序列复杂性与合理的化学衍生作用一起使用,以建立线性自由能关系(LFER),以研究肽的碰撞诱导解离(CID)。衍生化产生的肽组在N端具有不同的脂族氨基酸设计者残基,但对于自然存在且不受控制的其余序列保持不变。通过液相色谱多反应监测质谱法有利地监测衍生肽的碰撞碎裂。系统地调节肽N末端的气相碱性会建立LFER,这些LFER报告双质子化胰蛋白酶肽的所有主链酰胺的CID的结构相似性和差异。对于内部或C端酰胺的裂解,肽N端主要影响N端质子的迁移,而不是直接参与酰胺裂解。相反,末端残基在第一和第二酰胺键的裂解中起更明显的作用。用于第二酰胺键的对称和不对称裂解之间竞争的LFER支持N端片段的质子化恶唑酮结构。竞争受前三个残基的化学作用局部影响,而两个质子化位点之间的电荷排斥作用则受到远程影响。末端残基在第一和第二酰胺键的裂解中起更明显的作用。用于第二酰胺键的对称和不对称裂解之间竞争的LFER支持N端片段的质子化恶唑酮结构。竞争受前三个残基的化学作用局部影响,而两个质子化位点之间的电荷排斥作用则受到远程影响。末端残基在第一和第二酰胺键的裂解中起更明显的作用。用于第二酰胺键的对称和不对称裂解之间竞争的LFER支持N端片段的质子化恶唑酮结构。竞争受前三个残基的化学作用局部影响,而两个质子化位点之间的电荷排斥作用则受到远程影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号