当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Connection between Lithium Coordination and Lithium Diffusion in [Pyr12O1][FTFSI] Ionic Liquid Electrolytes
ChemSusChem ( IF 7.5 ) Pub Date : 2018-02-05 , DOI: 10.1002/cssc.201702288 Guinevere A. Giffin 1, 2, 3 , Arianna Moretti 1, 2 , Sangsik Jeong 1, 2 , Kartik Pilar 4, 5 , Marc Brinkkötter 6 , Steven G. Greenbaum 4, 5 , Monika Schönhoff 6 , Stefano Passerini 1, 2
ChemSusChem ( IF 7.5 ) Pub Date : 2018-02-05 , DOI: 10.1002/cssc.201702288 Guinevere A. Giffin 1, 2, 3 , Arianna Moretti 1, 2 , Sangsik Jeong 1, 2 , Kartik Pilar 4, 5 , Marc Brinkkötter 6 , Steven G. Greenbaum 4, 5 , Monika Schönhoff 6 , Stefano Passerini 1, 2
Affiliation
|
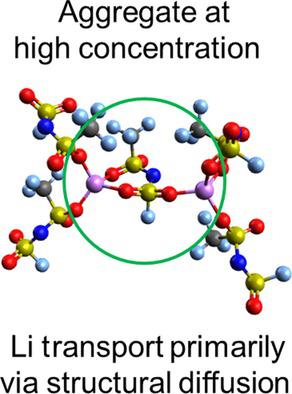
The use of highly concentrated ionic liquid‐based electrolytes results in improved rate capability and capacity retention at 20 °C compared to Li+‐dilute systems in Li‐metal and Li‐ion cells. This work explores the connection between the bulk electrolyte properties and the molecular organization to provide insight into the concentration dependence of the Li+ transport mechanisms. Below 30 mol %, the Li+‐containing species are primarily smaller complexes (one Li+ cation) and the Li+ ion transport is mostly derived from the vehicular transport. Above 30 mol %, where the viscosity is substantially higher and the conductivity lower, the Li+‐containing species are a mix of small and large complexes (one and more than one Li+ cation, respectively). The overall conduction mechanism likely changes to favor structural diffusion through the exchange of anions in the first Li+ solvation shell. The good rate performance is likely directly influenced by the presence of larger Li+ complexes, which promote Li+‐ion transport (as opposed to Li+‐complex transport) and increase the Li+ availability at the electrode.
中文翻译:

[Pyr12O1] [FTFSI]离子液体电解质中锂配位与锂扩散之间的联系
与锂金属和锂离子电池中的Li +稀体系相比,使用高浓度离子液体基电解质可提高倍率能力和20°C时的容量保持率。这项工作探索了整体电解质性能与分子组织之间的联系,以洞悉Li +传输机制对浓度的依赖性。低于30摩尔%,含Li +的物质主要是较小的络合物(一个Li +阳离子),并且Li +离子的迁移主要来自车辆迁移。高于30 mol%时,粘度显着较高而电导率较低,Li +包含的物种是大大小小的复合物(分别是一个和一个以上的Li +阳离子)的混合物。整个传导机制可能会发生变化,从而通过第一个Li +溶剂化壳中的阴离子交换而有利于结构扩散。良好的倍率性能很可能直接被较大Li的存在的影响+复合物,其促进锂+ -离子传输(相对于Li + -配合物运输)和增加的锂+在电极可用性。
更新日期:2018-02-05
中文翻译:

[Pyr12O1] [FTFSI]离子液体电解质中锂配位与锂扩散之间的联系
与锂金属和锂离子电池中的Li +稀体系相比,使用高浓度离子液体基电解质可提高倍率能力和20°C时的容量保持率。这项工作探索了整体电解质性能与分子组织之间的联系,以洞悉Li +传输机制对浓度的依赖性。低于30摩尔%,含Li +的物质主要是较小的络合物(一个Li +阳离子),并且Li +离子的迁移主要来自车辆迁移。高于30 mol%时,粘度显着较高而电导率较低,Li +包含的物种是大大小小的复合物(分别是一个和一个以上的Li +阳离子)的混合物。整个传导机制可能会发生变化,从而通过第一个Li +溶剂化壳中的阴离子交换而有利于结构扩散。良好的倍率性能很可能直接被较大Li的存在的影响+复合物,其促进锂+ -离子传输(相对于Li + -配合物运输)和增加的锂+在电极可用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号