当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2D and 3D lanthanide metal–organic frameworks constructed from three benzenedicarboxylate ligands: synthesis, structure and luminescent properties†
CrystEngComm ( IF 2.6 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1039/c7ce01773a Li-Xin You 1, 2, 3, 4 , Bai-Bei Zhao 1, 2, 3, 4 , Hui-Jie Liu 1, 2, 3, 4 , Shu-Ju Wang 1, 2, 3, 4 , Gang Xiong 1, 2, 3, 4 , Yong-Ke He 1, 2, 3, 4 , Fu Ding 1, 2, 3, 4 , Jonas J. Joos 5, 6, 7, 8, 9 , Philippe F. Smet 5, 6, 7, 8, 9 , Ya-Guang Sun 1, 2, 3, 4
CrystEngComm ( IF 2.6 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1039/c7ce01773a Li-Xin You 1, 2, 3, 4 , Bai-Bei Zhao 1, 2, 3, 4 , Hui-Jie Liu 1, 2, 3, 4 , Shu-Ju Wang 1, 2, 3, 4 , Gang Xiong 1, 2, 3, 4 , Yong-Ke He 1, 2, 3, 4 , Fu Ding 1, 2, 3, 4 , Jonas J. Joos 5, 6, 7, 8, 9 , Philippe F. Smet 5, 6, 7, 8, 9 , Ya-Guang Sun 1, 2, 3, 4
Affiliation
|
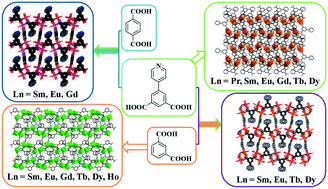
Nineteen lanthanide–benzenedicarboxylate complexes were obtained under similar hydrothermal conditions: [Ln(IP-Py)(HIP-Py)(H2O)2]n (Ln = Pr (1), Sm (2), Eu (3), Gd (4), Tb (5), Dy (6)), [Ln4(IP)6(H2O)4(DMF)·DMF·mH2O]n (Ln = Sm, m = 1 (7), Eu, m = 1.5 (8), Gd, m = 1.5 (9), Tb, m = 1.5 (10), Dy, m = 1 (11), Ho, m = 1.5 (12)), [Ln2(IP-Py)2(IP)(H2O)4·H2O]n (Ln = Sm (13), Eu (14), Tb (15), Dy (16)) and [Ln2(IP-Py)2(TP)(H2O)4·H2O]n (Ln = Sm (17), Eu (18), Gd (19)), where: H2IP-Py = 5-(4-pyridyl)-isophthalic acid), H2IP = isophthalic acid, H2TP = terephthalic acid. Complexes 1–6 exhibit a two-dimensional layered structure based on H2IP-Py. For 7–12, the Ln3+ ions are linked into one-dimensional chains with two triple-stranded helices of opposite handedness and further constructed into a two-dimensional layer by coordination of the IP2− ligands. For complexes 13–16 and 17–19, a similar two-dimensional layer was formed by coordination of Ln3+ and IP-Py2−, and three-dimensional pillar-layered frameworks were formed due to the layers bridged by the auxiliary IP2− or TP2− ligand, respectively. Most complexes show the characteristic narrow-banded lanthanide 4fN–4fN emission in combination with luminescence from the ligands, with the relative contribution depending on the energy transfer from the ligands to the lanthanides. The ligands play an important role in the sensitization of the lanthanide emission. The onset of the main excitation band for the Eu3+ complexes is found at the lowest energy for 14, enabling excitation by near-UV LEDs. The complexes containing the technologically relevant ions for (back) lighting applications, Tb3+ (green) and Eu3+ (red), are evaluated in detail in terms of luminescence lifetime and thermal stability. The emission intensity of selected Eu3+ and Tb3+ compounds is stable up to room temperature.
中文翻译:

由三个苯二甲酸配体构成的2D和3D镧系金属有机骨架:合成,结构和发光性能†
在相似的水热条件下获得了19种镧系元素-苯二甲酸络合物:[Ln(IP-Py)(HIP-Py)(H 2 O)2 ] n(Ln = Pr(1),Sm(2),Eu(3), Gd(4),Tb(5),Dy(6)),[Ln 4(IP)6(H 2 O)4(DMF)·DMF· m H 2 O] n(Ln = Sm,m = 1(7),Eu,m = 1.5(8),Gd,m = 1.5(9),Tb,m = 1.5(10),Dy,m = 1(11),Ho,m = 1.5(12)),[Ln 2(IP-Py)2(IP)(H 2 O)4 ·H 2 O] n(Ln = Sm(13),Eu(14),Tb(15),Dy(16))和[Ln 2(IP-Py)2(TP)(H 2 O)4 ·H 2 O ] n(Ln = Sm(17),Eu(18),Gd(19)),其中:H 2 IP-Py = 5-(4-吡啶基)-间苯二甲酸),H 2 IP =间苯二甲酸,H 2 TP =对苯二甲酸。配合物1-6显示基于H 2 IP-Py的二维分层结构。对于7–12,Ln 3+离子通过两个具有相反手性的三链螺旋连接成一维链,并通过IP 2-配体的配合进一步构建成二维层。对于复合物13–16和17–19,通过Ln 3+和IP-Py 2−的配位形成了类似的二维层,并且由于分别由辅助IP 2-或TP 2-配体桥接的层而形成了三维柱状层状框架。大多数配合物在结合配体的发光时都表现出特征性的窄带镧系元素4f N –4f N发射,其相对贡献取决于从配体到镧系元素的能量转移。配体在镧系元素发射的敏化中起重要作用。Eu 3+配合物的主激发带的起始能量最低,为14,可通过近紫外LED激发。包含与技术相关的离子的配合物,用于(逆向)照明应用,Tb根据发光寿命和热稳定性详细评估了3+(绿色)和Eu 3+(红色)。所选的Eu 3+和Tb 3+化合物的发射强度在室温下稳定。
更新日期:2017-12-27
中文翻译:

由三个苯二甲酸配体构成的2D和3D镧系金属有机骨架:合成,结构和发光性能†
在相似的水热条件下获得了19种镧系元素-苯二甲酸络合物:[Ln(IP-Py)(HIP-Py)(H 2 O)2 ] n(Ln = Pr(1),Sm(2),Eu(3), Gd(4),Tb(5),Dy(6)),[Ln 4(IP)6(H 2 O)4(DMF)·DMF· m H 2 O] n(Ln = Sm,m = 1(7),Eu,m = 1.5(8),Gd,m = 1.5(9),Tb,m = 1.5(10),Dy,m = 1(11),Ho,m = 1.5(12)),[Ln 2(IP-Py)2(IP)(H 2 O)4 ·H 2 O] n(Ln = Sm(13),Eu(14),Tb(15),Dy(16))和[Ln 2(IP-Py)2(TP)(H 2 O)4 ·H 2 O ] n(Ln = Sm(17),Eu(18),Gd(19)),其中:H 2 IP-Py = 5-(4-吡啶基)-间苯二甲酸),H 2 IP =间苯二甲酸,H 2 TP =对苯二甲酸。配合物1-6显示基于H 2 IP-Py的二维分层结构。对于7–12,Ln 3+离子通过两个具有相反手性的三链螺旋连接成一维链,并通过IP 2-配体的配合进一步构建成二维层。对于复合物13–16和17–19,通过Ln 3+和IP-Py 2−的配位形成了类似的二维层,并且由于分别由辅助IP 2-或TP 2-配体桥接的层而形成了三维柱状层状框架。大多数配合物在结合配体的发光时都表现出特征性的窄带镧系元素4f N –4f N发射,其相对贡献取决于从配体到镧系元素的能量转移。配体在镧系元素发射的敏化中起重要作用。Eu 3+配合物的主激发带的起始能量最低,为14,可通过近紫外LED激发。包含与技术相关的离子的配合物,用于(逆向)照明应用,Tb根据发光寿命和热稳定性详细评估了3+(绿色)和Eu 3+(红色)。所选的Eu 3+和Tb 3+化合物的发射强度在室温下稳定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号