当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Binding of Zn(II) to Tropomyosin Receptor Kinase A in Complex with Its Cognate Nerve Growth Factor: Insights from Molecular Simulation and in Vitro Essays
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acschemneuro.7b00470 Adriana Pietropaolo 1 , Antonio Magrì 2 , Valentina Greco 3 , Valeria Losasso 4, 5 , Diego La Mendola 6 , Sebastiano Sciuto 3 , Paolo Carloni 4, 5 , Enrico Rizzarelli 3
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acschemneuro.7b00470 Adriana Pietropaolo 1 , Antonio Magrì 2 , Valentina Greco 3 , Valeria Losasso 4, 5 , Diego La Mendola 6 , Sebastiano Sciuto 3 , Paolo Carloni 4, 5 , Enrico Rizzarelli 3
Affiliation
|
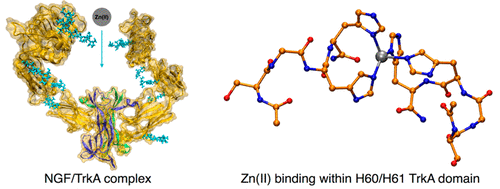
The binding of the human nerve growth factor (NGF) protein to tropomyosin receptor kinase A (TrkA) is associated with Alzhemeir’s development. Owing to the large presence of zinc(II) ions in the synaptic compartments, the zinc ions might be bound to the complex in vivo. Here, we have identified a putative zinc binding site using a combination of computations and experiments. First, we have predicted structural features of the NGF/TrkA complex in an aqueous solution by molecular simulation. Metadynamics free energy calculations suggest that these are very similar to those in the X-ray structure. Here, the “crab” structure of the NGF shape binds tightly to two TrkA “pincers”. Transient conformations of the complex include both more extended and more closed conformations. Interestingly, the latter features facial histidines (His60 and His61) among the N-terminal D1–D3 domains, each of which is a potential binding region for biometals. This suggests the presence of a four-His Zn binding site connecting the two chains. To address this issue, we investigated the binding of a D1–D3 domains’ peptide mimic by stability constant and nuclear magnetic resonance measurements, complemented by density functional theory-based calculations. Taken together, these establish unambiguously a four-His coordination of the metal ion in the model systems, supporting the presence of our postulated binding site in the NGF/TrkA complex.
中文翻译:

锌(II)结合其同源神经生长因子的结合中的肌球蛋白受体激酶A:从分子模拟和体外论文的见解。
人神经生长因子(NGF)蛋白与原肌球蛋白受体激酶A(TrkA)的结合与Alzhemeir的发展有关。由于突触区室中大量存在锌(II)离子,锌离子可能在体内与复合物结合。在这里,我们已经结合计算和实验确定了一个假定的锌结合位点。首先,我们通过分子模拟预测了NGF / TrkA复合物在水溶液中的结构特征。元动力学自由能计算表明,这些与X射线结构中的非常相似。在此,NGF形状的“螃蟹”结构与两个TrkA“夹子”紧密结合。复合物的瞬时构象包括更扩展的构象和更封闭的构象。有趣的是,后者在N末端D1-D3结构域中具有面部组氨酸(His60和His61),每个结构域都是生物金属的潜在结合区域。这表明存在连接两个链的四个His Zn结合位点。为了解决这个问题,我们通过稳定常数和核磁共振测量研究了D1-D3域肽模拟物的结合,并辅之以基于密度泛函理论的计算。综上所述,它们在模型系统中明确建立了金属离子的四-His配位,从而支持了我们假定的结合位点在NGF / TrkA复合物中的存在。
更新日期:2017-12-27
中文翻译:

锌(II)结合其同源神经生长因子的结合中的肌球蛋白受体激酶A:从分子模拟和体外论文的见解。
人神经生长因子(NGF)蛋白与原肌球蛋白受体激酶A(TrkA)的结合与Alzhemeir的发展有关。由于突触区室中大量存在锌(II)离子,锌离子可能在体内与复合物结合。在这里,我们已经结合计算和实验确定了一个假定的锌结合位点。首先,我们通过分子模拟预测了NGF / TrkA复合物在水溶液中的结构特征。元动力学自由能计算表明,这些与X射线结构中的非常相似。在此,NGF形状的“螃蟹”结构与两个TrkA“夹子”紧密结合。复合物的瞬时构象包括更扩展的构象和更封闭的构象。有趣的是,后者在N末端D1-D3结构域中具有面部组氨酸(His60和His61),每个结构域都是生物金属的潜在结合区域。这表明存在连接两个链的四个His Zn结合位点。为了解决这个问题,我们通过稳定常数和核磁共振测量研究了D1-D3域肽模拟物的结合,并辅之以基于密度泛函理论的计算。综上所述,它们在模型系统中明确建立了金属离子的四-His配位,从而支持了我们假定的结合位点在NGF / TrkA复合物中的存在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号