当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluorination Enhances Serum Stability of Bioreducible Poly(amido amine) Polyplexes and Enables Efficient Intravenous siRNA Delivery
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2017-12-27 , DOI: 10.1002/adhm.201700978 Gang Chen 1 , Kaikai Wang 1 , Yixin Wang 1 , Pengkai Wu 1 , Minjie Sun 1 , David Oupický 1, 2
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2017-12-27 , DOI: 10.1002/adhm.201700978 Gang Chen 1 , Kaikai Wang 1 , Yixin Wang 1 , Pengkai Wu 1 , Minjie Sun 1 , David Oupický 1, 2
Affiliation
|
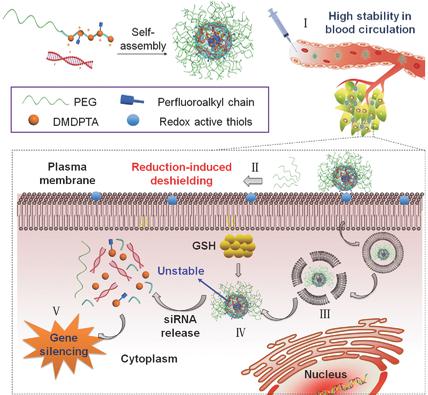
The use of small interfering RNA (siRNA) in cancer treatment has been limited by the lack of effective systemic delivery methods. Although synthetic polycations have been widely explored in siRNA delivery, polycation/siRNA polyplexes often suffer from insufficient stability in vivo. Here, rationally designed siRNA delivery systems that meet the requirements for systemic siRNA delivery to distant tumors are reported. The hypothesis that modular design of delivery systems based on poly(amido amine)s that combine fluorination for systemic stability with bioreducibility for easy intracellular siRNA release, and PEGylation for improved safety and colloidal stability will overcome problems with contradicting siRNA delivery demands is tested. PEGylated, fluorinated, and bioreducible copolymers (PEG‐PCD‐F) with different degree of fluorination are thus synthesized. The fluorinated copolymers readily formed polyplexes with siRNA and achieved greatly improved gene silencing efficacy in multiple cell lines in vitro when compared with nonfluorinated controls. The results show fluorination‐induced enhancement of stability, cellular uptake, and endosomal escape of the polyplexes, while exhibiting efficient siRNA release in reducing intracellular environment. PEG‐PCD‐F polyplexes with siRNA against Bcl2 inhibit breast tumor growth following systemic intravenous administration. The results provide strong evidence of successful combination of bioreducibility with fluorination and PEGylation to achieve systemic siRNA polyplex delivery.
中文翻译:

氟化作用增强了可生物还原的聚(酰胺基胺)多聚体的血清稳定性,并使静脉内siRNA的有效递送成为可能
由于缺乏有效的全身递送方法,小干扰RNA(siRNA)在癌症治疗中的应用受到了限制。尽管合成的聚阳离子已经在siRNA输送中得到了广泛的探索,但是聚阳离子/ siRNA的复合物通常在体内缺乏足够的稳定性。在此,报告了合理设计的siRNA递送系统,该系统可满足将siRNA全身递送至远处肿瘤的要求。验证了基于聚(酰胺胺)的递送系统的模块化设计的假设,该设计将氟化用于系统稳定性和生物还原性相结合,以使细胞内siRNA易于释放,而聚乙二醇化可提高安全性和胶体稳定性,从而克服了与siRNA递送需求矛盾的问题。聚乙二醇化,氟化,从而合成了具有不同氟化度的生物可还原共聚物(PEG-PCD-F)。含氟共聚物与siRNA容易形成多链体,与非含氟对照相比,在体外多个细胞系中实现了大大提高的基因沉默效果。结果显示氟化诱导的稳定性,多聚体的细胞摄取和内体逃逸的增强,同时在减少细胞内环境中表现出有效的siRNA释放。带有针对Bcl2的siRNA的PEG-PCD-F多聚体可抑制全身静脉内给药后乳腺肿瘤的生长。该结果提供了生物还原性与氟化和聚乙二醇化成功结合以实现全身性siRNA多聚体递送的有力证据。含氟共聚物与siRNA容易形成多链体,与非含氟对照相比,在体外多个细胞系中实现了大大提高的基因沉默效果。结果显示氟化诱导的稳定性,多聚体的细胞摄取和内体逃逸的增强,同时在减少细胞内环境中表现出有效的siRNA释放。带有针对Bcl2的siRNA的PEG-PCD-F多聚体可抑制全身静脉内给药后乳腺肿瘤的生长。该结果提供了生物还原性与氟化和聚乙二醇化成功结合以实现全身性siRNA多聚体递送的有力证据。与未氟化的对照相比,该氟化的共聚物易于与siRNA形成多链体,并在体外多个细胞系中实现了大大提高的基因沉默功效。结果显示氟化诱导的稳定性,多聚体的细胞摄取和内体逃逸的增强,同时在减少细胞内环境中表现出有效的siRNA释放。带有针对Bcl2的siRNA的PEG-PCD-F多聚体可抑制全身静脉内给药后乳腺肿瘤的生长。该结果提供了生物还原性与氟化和聚乙二醇化成功结合以实现全身性siRNA多聚体递送的有力证据。细胞摄取和多聚体的内体逃逸,同时在减少的细胞内环境中表现出有效的siRNA释放。带有针对Bcl2的siRNA的PEG-PCD-F多聚体可抑制全身静脉内给药后乳腺肿瘤的生长。该结果提供了生物还原性与氟化和聚乙二醇化成功结合以实现全身性siRNA多聚体递送的有力证据。细胞摄取和多聚体的内体逃逸,同时在减少的细胞内环境中表现出有效的siRNA释放。带有针对Bcl2的siRNA的PEG-PCD-F多聚体可抑制全身静脉内给药后乳腺肿瘤的生长。该结果提供了生物还原性与氟化和聚乙二醇化成功结合以实现全身性siRNA多聚体递送的有力证据。
更新日期:2017-12-27
中文翻译:

氟化作用增强了可生物还原的聚(酰胺基胺)多聚体的血清稳定性,并使静脉内siRNA的有效递送成为可能
由于缺乏有效的全身递送方法,小干扰RNA(siRNA)在癌症治疗中的应用受到了限制。尽管合成的聚阳离子已经在siRNA输送中得到了广泛的探索,但是聚阳离子/ siRNA的复合物通常在体内缺乏足够的稳定性。在此,报告了合理设计的siRNA递送系统,该系统可满足将siRNA全身递送至远处肿瘤的要求。验证了基于聚(酰胺胺)的递送系统的模块化设计的假设,该设计将氟化用于系统稳定性和生物还原性相结合,以使细胞内siRNA易于释放,而聚乙二醇化可提高安全性和胶体稳定性,从而克服了与siRNA递送需求矛盾的问题。聚乙二醇化,氟化,从而合成了具有不同氟化度的生物可还原共聚物(PEG-PCD-F)。含氟共聚物与siRNA容易形成多链体,与非含氟对照相比,在体外多个细胞系中实现了大大提高的基因沉默效果。结果显示氟化诱导的稳定性,多聚体的细胞摄取和内体逃逸的增强,同时在减少细胞内环境中表现出有效的siRNA释放。带有针对Bcl2的siRNA的PEG-PCD-F多聚体可抑制全身静脉内给药后乳腺肿瘤的生长。该结果提供了生物还原性与氟化和聚乙二醇化成功结合以实现全身性siRNA多聚体递送的有力证据。含氟共聚物与siRNA容易形成多链体,与非含氟对照相比,在体外多个细胞系中实现了大大提高的基因沉默效果。结果显示氟化诱导的稳定性,多聚体的细胞摄取和内体逃逸的增强,同时在减少细胞内环境中表现出有效的siRNA释放。带有针对Bcl2的siRNA的PEG-PCD-F多聚体可抑制全身静脉内给药后乳腺肿瘤的生长。该结果提供了生物还原性与氟化和聚乙二醇化成功结合以实现全身性siRNA多聚体递送的有力证据。与未氟化的对照相比,该氟化的共聚物易于与siRNA形成多链体,并在体外多个细胞系中实现了大大提高的基因沉默功效。结果显示氟化诱导的稳定性,多聚体的细胞摄取和内体逃逸的增强,同时在减少细胞内环境中表现出有效的siRNA释放。带有针对Bcl2的siRNA的PEG-PCD-F多聚体可抑制全身静脉内给药后乳腺肿瘤的生长。该结果提供了生物还原性与氟化和聚乙二醇化成功结合以实现全身性siRNA多聚体递送的有力证据。细胞摄取和多聚体的内体逃逸,同时在减少的细胞内环境中表现出有效的siRNA释放。带有针对Bcl2的siRNA的PEG-PCD-F多聚体可抑制全身静脉内给药后乳腺肿瘤的生长。该结果提供了生物还原性与氟化和聚乙二醇化成功结合以实现全身性siRNA多聚体递送的有力证据。细胞摄取和多聚体的内体逃逸,同时在减少的细胞内环境中表现出有效的siRNA释放。带有针对Bcl2的siRNA的PEG-PCD-F多聚体可抑制全身静脉内给药后乳腺肿瘤的生长。该结果提供了生物还原性与氟化和聚乙二醇化成功结合以实现全身性siRNA多聚体递送的有力证据。









































 京公网安备 11010802027423号
京公网安备 11010802027423号