当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal Structures and Inhibitor Interactions of Mouse and Dog MTH1 Reveal Species-Specific Differences in Affinity
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.biochem.7b01163 Mohit Narwal 1 , Ann-Sofie Jemth 2 , Robert Gustafsson 1 , Ingrid Almlöf 2 , Ulrika Warpman Berglund 2 , Thomas Helleday 2 , Pål Stenmark 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.biochem.7b01163 Mohit Narwal 1 , Ann-Sofie Jemth 2 , Robert Gustafsson 1 , Ingrid Almlöf 2 , Ulrika Warpman Berglund 2 , Thomas Helleday 2 , Pål Stenmark 1
Affiliation
|
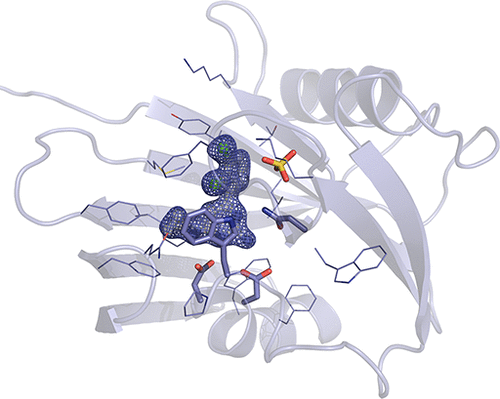
MTH1 hydrolyzes oxidized nucleoside triphosphates, thereby sanitizing the nucleotide pool from oxidative damage. This prevents incorporation of damaged nucleotides into DNA, which otherwise would lead to mutations and cell death. The high level of reactive oxygen species in cancer cells leads to a higher level of oxidized nucleotides in cancer cells compared to that in nonmalignant cells, making cancer cells more dependent on MTH1 for survival. The possibility of specifically targeting cancer cells by inhibiting MTH1 has highlighted MTH1 as a promising cancer target. The progression of MTH1 inhibitors into the clinic requires animal studies, and knowledge of species differences in the potency of inhibitors is vitally important. We here show that the human MTH1 inhibitor TH588 is approximately 20-fold less potent with respect to inhibition of mouse MTH1 than the human, rat, pig, and dog MTH1 proteins are. We present the crystal structures of mouse MTH1 in complex with TH588 and dog MTH1 and elucidate the structural and sequence basis for the observed difference in affinity for TH588. We identify amino acid residue 116 in MTH1 as an important determinant of TH588 affinity. Furthermore, we present the structure of mouse MTH1 in complex with the substrate 8-oxo-dGTP. The crystal structures provide insight into the high degree of structural conservation between MTH1 proteins from different organisms and provide a detailed view of interactions between MTH1 and the inhibitor, revealing that minute structural differences can have a large impact on affinity and specificity.
中文翻译:

小鼠和狗MTH1的晶体结构和抑制剂相互作用揭示亲和力的物种特异性差异。
MTH1水解氧化的三磷酸核苷,从而使核苷酸库免受氧化损伤。这样可以防止将损坏的核苷酸掺入DNA中,否则将导致突变和细胞死亡。与非恶性细胞相比,癌细胞中高水平的活性氧导致癌细胞中的氧化核苷酸水平更高,从而使癌细胞更依赖于MTH1存活。通过抑制MTH1特异性靶向癌细胞的可能性突显了MTH1作为有希望的癌症靶标。MTH1抑制剂进入临床的过程需要进行动物研究,了解抑制剂效力方面的物种差异至关重要。我们在这里显示,人MTH1抑制剂TH588对小鼠MTH1的抑制作用比人,大鼠,猪和狗MTH1蛋白的抑制作用低约20倍。我们提出与TH588和狗MTH1配合的小鼠MTH1的晶体结构,并阐明观察到的对TH588亲和力差异的结构和序列基础。我们确定MTH1中的氨基酸残基116是TH588亲和力的重要决定因素。此外,我们提出了与底物8-oxo-dGTP复合的小鼠MTH1的结构。晶体结构可洞察来自不同生物体的MTH1蛋白之间的高度结构保守性,并提供MTH1与抑制剂之间相互作用的详细视图,从而揭示出微小的结构差异可对亲和力和特异性产生重大影响。
更新日期:2018-01-16
中文翻译:

小鼠和狗MTH1的晶体结构和抑制剂相互作用揭示亲和力的物种特异性差异。
MTH1水解氧化的三磷酸核苷,从而使核苷酸库免受氧化损伤。这样可以防止将损坏的核苷酸掺入DNA中,否则将导致突变和细胞死亡。与非恶性细胞相比,癌细胞中高水平的活性氧导致癌细胞中的氧化核苷酸水平更高,从而使癌细胞更依赖于MTH1存活。通过抑制MTH1特异性靶向癌细胞的可能性突显了MTH1作为有希望的癌症靶标。MTH1抑制剂进入临床的过程需要进行动物研究,了解抑制剂效力方面的物种差异至关重要。我们在这里显示,人MTH1抑制剂TH588对小鼠MTH1的抑制作用比人,大鼠,猪和狗MTH1蛋白的抑制作用低约20倍。我们提出与TH588和狗MTH1配合的小鼠MTH1的晶体结构,并阐明观察到的对TH588亲和力差异的结构和序列基础。我们确定MTH1中的氨基酸残基116是TH588亲和力的重要决定因素。此外,我们提出了与底物8-oxo-dGTP复合的小鼠MTH1的结构。晶体结构可洞察来自不同生物体的MTH1蛋白之间的高度结构保守性,并提供MTH1与抑制剂之间相互作用的详细视图,从而揭示出微小的结构差异可对亲和力和特异性产生重大影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号