当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study of Förster Resonance Energy Transfer to Lipid Domain Markers Ascertains Partitioning of Semisynthetic Lipidated N-Ras in Lipid Raft Nanodomains
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.biochem.7b01181 Anna K. Shishina 1 , Elizaveta A. Kovrigina 1 , Azamat R. Galiakhmetov 1 , Rajendra Rathore 1 , Evgenii L. Kovrigin 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.biochem.7b01181 Anna K. Shishina 1 , Elizaveta A. Kovrigina 1 , Azamat R. Galiakhmetov 1 , Rajendra Rathore 1 , Evgenii L. Kovrigin 1
Affiliation
|
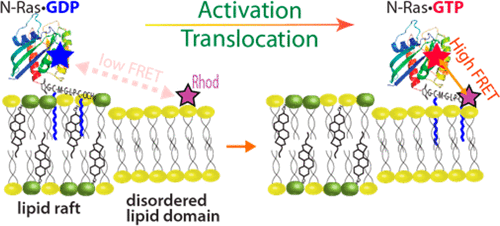
Cellular membranes are heterogeneous planar lipid bilayers displaying lateral phase separation with the nanometer-scale liquid-ordered phase (also known as “lipid rafts”) surrounded by the liquid-disordered phase. Many membrane-associated proteins were found to permanently integrate into the lipid rafts, which is critical for their biological function. Isoforms H and N of Ras GTPase possess a unique ability to switch their lipid domain preference depending on the type of bound guanine nucleotide (GDP or GTP). This behavior, however, has never been demonstrated in vitro in model bilayers with recombinant proteins and therefore has been attributed to the action of binding of Ras to other proteins at the membrane surface. In this paper, we report the observation of the nucleotide-dependent switch of lipid domain preferences of the semisynthetic lipidated N-Ras in lipid raft vesicles in the absence of additional proteins. To detect segregation of Ras molecules in raft and disordered lipid domains, we measured Förster resonance energy transfer between the donor fluorophore, mant, attached to the protein-bound guanine nucleotides, and the acceptor, rhodamine-conjugated lipid, localized into the liquid-disordered domains. Herein, we established that N-Ras preferentially populated raft domains when bound to mant-GDP, while losing its preference for rafts when it was associated with a GTP mimic, mant-GppNHp. At the same time, the isolated lipidated C-terminal peptide of N-Ras was found to be localized outside of the liquid-ordered rafts, most likely in the bulk-disordered lipid. Substitution of the N-terminal G domain of N-Ras with a homologous G domain of H-Ras disrupted the nucleotide-dependent lipid domain switch.
中文翻译:

Förster共振能量转移至脂质域标记的研究确定半合成脂质N-Ras在脂质筏纳米域中的分配。
细胞膜是异质的平面脂质双层,表现出横向相分离,纳米级液相有序相(也称为“脂筏”)被液相无序相包围。发现许多与膜相关的蛋白质永久整合到脂质筏中,这对它们的生物学功能至关重要。Ras GTPase的同工型H和N具有独特的能力,可根据结合的鸟嘌呤核苷酸的类型(GDP或GTP)切换其脂质结构域偏好。但是,这种行为从未在体外得到证实在具有重组蛋白的双层模型中,Raf被认为是由于Ras与膜表面其他蛋白的结合作用所致。在本文中,我们报告了在不存在其他蛋白质的情况下,脂筏囊泡中半合成脂化N-Ras的脂质结构域偏好的核苷酸依赖性开关的观察结果。为了检测Ras分子在筏和无序脂质域中的分离,我们测量了Förster共振能量转移,该能量在附于蛋白结合鸟嘌呤核苷酸的供体荧光团mant和定位在液体无序的受体罗丹明缀合的脂质之间域。在这里,我们确定N-Ras绑定到mant-GDP时优先填充筏域,而当它与GTP模仿mant-GppNHp相关联时就失去了对筏的偏好。同时,发现分离的N-Ras的脂化C末端肽位于液体有序筏的外部,最有可能位于大量无序的脂质中。用H-Ras的同源G结构域取代N-Ras的N-末端G结构域破坏了核苷酸依赖性脂质结构域开关。
更新日期:2018-01-10
中文翻译:

Förster共振能量转移至脂质域标记的研究确定半合成脂质N-Ras在脂质筏纳米域中的分配。
细胞膜是异质的平面脂质双层,表现出横向相分离,纳米级液相有序相(也称为“脂筏”)被液相无序相包围。发现许多与膜相关的蛋白质永久整合到脂质筏中,这对它们的生物学功能至关重要。Ras GTPase的同工型H和N具有独特的能力,可根据结合的鸟嘌呤核苷酸的类型(GDP或GTP)切换其脂质结构域偏好。但是,这种行为从未在体外得到证实在具有重组蛋白的双层模型中,Raf被认为是由于Ras与膜表面其他蛋白的结合作用所致。在本文中,我们报告了在不存在其他蛋白质的情况下,脂筏囊泡中半合成脂化N-Ras的脂质结构域偏好的核苷酸依赖性开关的观察结果。为了检测Ras分子在筏和无序脂质域中的分离,我们测量了Förster共振能量转移,该能量在附于蛋白结合鸟嘌呤核苷酸的供体荧光团mant和定位在液体无序的受体罗丹明缀合的脂质之间域。在这里,我们确定N-Ras绑定到mant-GDP时优先填充筏域,而当它与GTP模仿mant-GppNHp相关联时就失去了对筏的偏好。同时,发现分离的N-Ras的脂化C末端肽位于液体有序筏的外部,最有可能位于大量无序的脂质中。用H-Ras的同源G结构域取代N-Ras的N-末端G结构域破坏了核苷酸依赖性脂质结构域开关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号