当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A High-Performance Nanoreactor for Carbon–Oxygen Bond Hydrogenation Reactions Achieved by the Morphology of Nanotube-Assembled Hollow Spheres
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acscatal.7b03026 Dawei Yao 1 , Yue Wang 1 , Ying Li 1 , Yujun Zhao 1 , Jing Lv 1 , Xinbin Ma 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acscatal.7b03026 Dawei Yao 1 , Yue Wang 1 , Ying Li 1 , Yujun Zhao 1 , Jing Lv 1 , Xinbin Ma 1
Affiliation
|
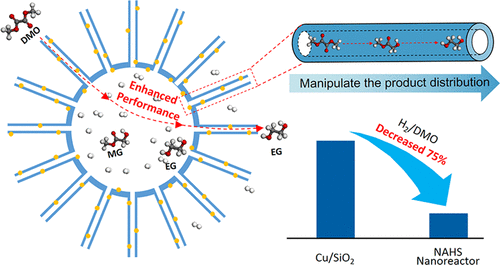
Hydrogenation of carbon–oxygen bonds is extensively used in organic synthesis. However, a high partial pressure of hydrogen or the presence of excess hydrogen is usually essential to achieve favorable conversions. In addition, because most hydrogenations are consecutive reactions, the selectivity is difficult to manipulate, leading to an unsatisfactory distribution of products. Herein, a copper silicate nanoreactor with a nanotube-assembled hollow sphere (NAHS) hierarchical structure is proposed as a solution to these problems. In the case of dimethyl oxalate (DMO) hydrogenation, the NAHS nanoreactor achieves remarkable catalytic activity (the yield of ethylene glycol is 95%) and stability (>300 h) when the H2/DMO molar ratio is as low as 20 (in comparison to typical values of 80–200). For further investigation, nanotubes and lamellar-shaped Cu/SiO2 catalysts with similar surface areas of active sites of NAHSs were investigated as contrasts. By a combination of high-pressure hydrogen adsorption and Monte Carlo simulation, it is demonstrated that hydrogen can be enriched on the concave surface of nanotubes and hollow spheres, leading to a favorable activity in such a low H2 proportion. Furthermore, because of the spatial restriction effect of reactants, adjusting the diffusion path is an effective route for manipulating the selectivity and product distribution of the hydrogenation reactions. By variation in the length of nanotubes on NAHS, the yields of methyl glycolate and ethylene glycol are easily controlled. The NAHS nanoreactor, with insights into the effect of morphology on hydrogen enrichment and spatial restriction of reactant diffusion, offers inspiring possibilities in the rational design of catalysts for hydrogenation reactions.
中文翻译:

纳米管组装空心球的形貌实现的高性能碳-氧键加氢反应纳米反应器
碳-氧键的加氢广泛用于有机合成中。然而,氢的高分压或过量氢的存在通常对于实现有利的转化是必不可少的。另外,由于大多数氢化是连续反应,因此选择性难以操作,导致产物分布不令人满意。在本文中,提出了具有纳米管组装的空心球(NAHS)分级结构的硅酸铜纳米反应器作为解决这些问题的方案。在草酸二甲酯(DMO)加氢的情况下,当H 2时,NAHS纳米反应器具有出色的催化活性(乙二醇的收率为95%)和稳定性(> 300 h)。/ DMO摩尔比低至20(与典型值80–200相比)。为了进一步研究,研究了具有相似的NAHS活性部位表面积的纳米管和层状Cu / SiO 2催化剂作为对比。通过高压氢吸附和蒙特卡罗模拟的结合,证明了氢可以富集在纳米管和中空球体的凹表面上,从而在如此低的H 2中产生有利的活性。部分。此外,由于反应物的空间限制作用,调节扩散路径是用于控制氢化反应的选择性和产物分布的有效途径。通过改变NAHS上纳米管的长度,可以轻松控制乙醇酸甲酯和乙二醇的收率。NAHS纳米反应器深入研究了形态对氢富集的影响以及反应物扩散的空间限制,为合理设计加氢反应催化剂提供了启发性的可能性。
更新日期:2018-01-17
中文翻译:

纳米管组装空心球的形貌实现的高性能碳-氧键加氢反应纳米反应器
碳-氧键的加氢广泛用于有机合成中。然而,氢的高分压或过量氢的存在通常对于实现有利的转化是必不可少的。另外,由于大多数氢化是连续反应,因此选择性难以操作,导致产物分布不令人满意。在本文中,提出了具有纳米管组装的空心球(NAHS)分级结构的硅酸铜纳米反应器作为解决这些问题的方案。在草酸二甲酯(DMO)加氢的情况下,当H 2时,NAHS纳米反应器具有出色的催化活性(乙二醇的收率为95%)和稳定性(> 300 h)。/ DMO摩尔比低至20(与典型值80–200相比)。为了进一步研究,研究了具有相似的NAHS活性部位表面积的纳米管和层状Cu / SiO 2催化剂作为对比。通过高压氢吸附和蒙特卡罗模拟的结合,证明了氢可以富集在纳米管和中空球体的凹表面上,从而在如此低的H 2中产生有利的活性。部分。此外,由于反应物的空间限制作用,调节扩散路径是用于控制氢化反应的选择性和产物分布的有效途径。通过改变NAHS上纳米管的长度,可以轻松控制乙醇酸甲酯和乙二醇的收率。NAHS纳米反应器深入研究了形态对氢富集的影响以及反应物扩散的空间限制,为合理设计加氢反应催化剂提供了启发性的可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号