当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extraction of Gd3+ and UO22+ Ions Using Polystyrene Grafted Dibenzo Crown Ether (DB18C6) with Octanol and Nitrobenzene: A Molecular Dynamics Study
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jpcb.7b11384 Praveenkumar Sappidi 1 , Sadanandam Namsani 1 , Sk. Musharaf Ali 2 , Jayant Kumar Singh 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jpcb.7b11384 Praveenkumar Sappidi 1 , Sadanandam Namsani 1 , Sk. Musharaf Ali 2 , Jayant Kumar Singh 1
Affiliation
|
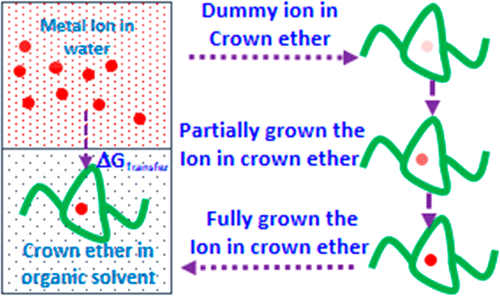
Atomistic molecular dynamics (MD) simulations are performed in order to derive thermodynamic properties important to understand the extraction of gadolinium (Gd3+) and uranium dioxide (UO2) with dibenzo crown ether (DBCE) in nitrobenzene (NB) and octanol (OCT) solvents. The effect of polystyrene graft length, on DBCE, on the binding behavior of Gd3+ and UO22+ is investigated for the first time. Our simulation results demonstrate that the binding of Gd3+ and UO22+ onto the oxygens of crown ethers is favorable for polystyrene grafted crown ether in the organic solvents OCT and NB. The metal ion binding free energy (ΔGBinding) in different solvent environments is calculated using the thermodynamic integration (TI) method. ΔGBinding becomes more favorable in both solvents, NB and OCT, with an increase in the polystyrene monomer length. The metal ion transferability from an aqueous phase to an organic phase is estimated by calculating transfer free-energy calculations (ΔGTransfer). ΔGTransfer is significantly favorable for both Gd3+ and UO22+ for the transfer from the aqueous phase to the organic phase (i.e., NB and OCT) via ion-complexation to DBCE with an increase in polystyrene length. The partition coefficient (log P) values for Gd3+ and UO22+ show a 5-fold increase in separation capacity with polystyrene grafted DBCE. We corroborate the observed behavior by further analyzing the structural and dynamical properties of the ions in different phases.
中文翻译:

辛醇和硝基苯的聚苯乙烯接枝二苯并冠醚(DB18C6)萃取Gd 3+和UO 2 2+离子的分子动力学研究
为了获得热力学性质,进行原子分子动力学(MD)模拟对于理解二苯并冠醚(DBCE)在硝基苯(NB)和辛醇(OCT)中萃取g (Gd 3+)和二氧化铀(UO 2)具有重要意义。)溶剂。首次研究了聚苯乙烯接枝长度对DBCE对Gd 3+和UO 2 2+结合行为的影响。我们的模拟结果表明,在有机溶剂OCT和NB中,Gd 3+和UO 2 2+结合到冠醚的氧上对于聚苯乙烯接枝冠醚是有利的。所述金属离子结合自由能(Δ ģ结合在不同的溶剂环境中)是使用热力学整合(TI)方法来计算。Δ ģ结合成为两种溶剂,NB和OCT更有利的,与增加的聚苯乙烯单体的长度。从水相到有机相中的金属离子的转印是通过计算转移自由能的计算(估计Δ ģ转移)。Δ ģ转移为两个GD显著有利3+和UO 2 2+,用于经由离子络合从水相转移到有机相中(即,NB和OCT)到DBCE的增加聚苯乙烯长度。分配系数(log P)Gd 3+和UO 2 2+的值表明,聚苯乙烯接枝DBCE的分离能力提高了5倍。通过进一步分析离子在不同相中的结构和动力学性质,我们证实了观察到的行为。
更新日期:2018-01-16
中文翻译:

辛醇和硝基苯的聚苯乙烯接枝二苯并冠醚(DB18C6)萃取Gd 3+和UO 2 2+离子的分子动力学研究
为了获得热力学性质,进行原子分子动力学(MD)模拟对于理解二苯并冠醚(DBCE)在硝基苯(NB)和辛醇(OCT)中萃取g (Gd 3+)和二氧化铀(UO 2)具有重要意义。)溶剂。首次研究了聚苯乙烯接枝长度对DBCE对Gd 3+和UO 2 2+结合行为的影响。我们的模拟结果表明,在有机溶剂OCT和NB中,Gd 3+和UO 2 2+结合到冠醚的氧上对于聚苯乙烯接枝冠醚是有利的。所述金属离子结合自由能(Δ ģ结合在不同的溶剂环境中)是使用热力学整合(TI)方法来计算。Δ ģ结合成为两种溶剂,NB和OCT更有利的,与增加的聚苯乙烯单体的长度。从水相到有机相中的金属离子的转印是通过计算转移自由能的计算(估计Δ ģ转移)。Δ ģ转移为两个GD显著有利3+和UO 2 2+,用于经由离子络合从水相转移到有机相中(即,NB和OCT)到DBCE的增加聚苯乙烯长度。分配系数(log P)Gd 3+和UO 2 2+的值表明,聚苯乙烯接枝DBCE的分离能力提高了5倍。通过进一步分析离子在不同相中的结构和动力学性质,我们证实了观察到的行为。










































 京公网安备 11010802027423号
京公网安备 11010802027423号