Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-12-22 , DOI: 10.1016/j.mcat.2017.12.001 Raghavendra Shavi , Vishwanath Hiremath , Jeong Gil Seo
|
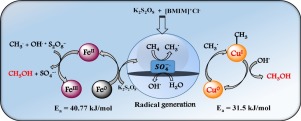
Oxidative conversion of methane gas into value-added chemicals such as methanol is of great interest due to high economic feasibility of liquid fuel molecules for storage and transportation purpose. Activation and conversion of methane occur at very high temperatures due to its strong CH bonding and hence the process is highly energy intensive. Therefore, homolytic cleavage of methane to produce CH3 and H radicals and subsequent conversion to methanol could be an alternative way to catalyze the reaction through a less energy-intensive process. In this work, radical-based conversion of methane to methanol was conducted in water-diluted 1-butyl-3-methylimidazolium chloride ionic liquid (IL) using metallic iron and copper as catalysts. The acidic IL, besides producing the high oxidation potential radicals from K2S2O8, enhanced their longevity. ZV Cu was found to be highly active in the reaction catalyzing with steady rate at a lower activation energy (Ea = 31.5 kJ/mol) and retains its oxidation state even after the reaction. On the other hand, ZV Fe, catalyzed the reaction with slightly slow initial rate ultimately resulting in moderate activation energy (40.77 kJ/mol). However, it was observed that ZV Fe fails to retain its oxidation state after reaction.
中文翻译:

金属铁和铜催化剂上甲烷自由基引发的甲烷氧化转化为甲醇
由于用于储存和运输目的的液体燃料分子的经济上的高度可行性,将甲烷气体氧化转化成增值的化学品(例如甲醇)备受关注。甲烷的活化和转化由于其强大的C H键而在非常高的温度下发生,因此该过程的能源消耗很高。因此,甲烷的均相裂解产生CH 3H自由基和随后的转化为甲醇可能是通过较少能量消耗的过程催化反应的另一种方法。在这项工作中,使用金属铁和铜作为催化剂,在水稀释的1-丁基-3-甲基咪唑鎓氯化物离子液体(IL)中,将甲烷自由基转化为甲醇。酸性IL除了从K 2 S 2 O 8中产生高氧化电位的自由基外,还提高了它们的寿命。发现ZV Cu在较低的活化能下以稳定的速率在反应催化中具有很高的活性(E a = 31.5kJ / mol),甚至在反应后仍保持其氧化态。另一方面,ZV Fe以稍慢的初始速率催化反应,最终产生适度的活化能(40.77 kJ / mol)。然而,观察到ZV Fe在反应后不能保持其氧化态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号