Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-12-22 , DOI: 10.1016/j.mcat.2017.11.040 Natália Marozsán , Henrietta Horváth , Éva Kováts , Antal Udvardy , Anikó Erdei , Mihály Purgel , Ferenc Joó
|
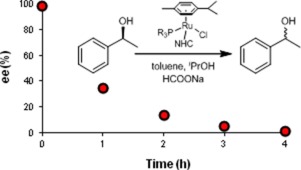
Five new complexes of the type [RuCl2(NHC)(η6-arene)] (4, 5, and 6) and [RuCl(NHC)(η6-arene)(PR3)]Cl (7 and 8) (NHCN-heterocyclic carbene = bmim, emim; arene = benzene, p-cymene; PR3 = PPh3 or pta = 1,3,5-triaza-7-phosphaadamantane) were synthetized and applied as catalysts (together with the known [RuCl2(bmim)(η6-p-cymene)] (3) with and without added PPh3) in racemization of optically active secondary alcohols in toluene. The highest catalytic activity, TOF = 9.3 h−1 (ee as low as 1.3% in 4 h at 95 °C) was observed in racemization of (S)-1-phenylethanol with a catalyst (4 mol%) prepared in situ from 3 and 1 equivalent of PPh3. It is of practical significance that formation of acetophenone byproduct was suppressed to 3.5% by 17% v/v isopropanol in toluene. DFT calculations revealed that the rate determining step in the suggested reaction mechanism was the agostic coordination of hydrogen on the chiral carbon atom of the alcohol substrate.
中文翻译:

新型(芳烃)Ru(II)-NHC和(芳烃)Ru(II)-NHC-叔膦配合物对仲醇的催化外消旋作用
的类型的五个新的复合物将[RuCl 2(NHC)(η 6 -arene)](4,5,和6)和将[RuCl(NHC)(η 6 -arene)(PR 3)] Cl(上7和8)合成了(NHC N-杂环卡宾= bmim,emim;芳烃=苯,对-异丙基; PR 3 = PPh 3或pta = 1,3,5-三氮杂-7-磷酸金刚烷)并用作催化剂(与已知方法一起使用)将[RuCl 2(BMIM)(η 6 - p -cymene)](3)有和没有加入PPH 3)光学活性仲醇在甲苯中的外消旋作用。在(S)-1-苯基乙醇与原位制备的催化剂(4 mol%)外消旋化中观察到最高的催化活性TOF = 9.3 h -1(ee在95°C下4 h时低至1.3 %)3和1当量的PPh 3。具有实际意义的是,甲苯中的17%v / v异丙醇将苯乙酮副产物的生成抑制到3.5%。DFT计算表明,建议反应机理中的速率确定步骤是氢在醇底物手性碳原子上的配位。









































 京公网安备 11010802027423号
京公网安备 11010802027423号