Molecular Catalysis ( IF 3.9 ) Pub Date : 2017-12-22 , DOI: 10.1016/j.mcat.2017.11.025 Ali Estejab , Gerardine G. Botte
|
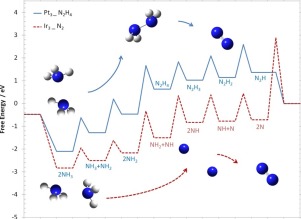
Density functional theory was performed on Pt3-xIrx (x = 0–3) clusters to investigate the ammonia electro-oxidation reaction to nitrogen. The adsorption of N2H4-x (x = 0–3) and the effect of cluster composition on the adsorption were investigated in Gaussian 09. N and NH were found to be the most stable intermediates on these clusters, with a pronounced stability caused by the presence of iridium. On a Pt cluster, the ammonia oxidation mechanism involves hydrazine formation followed by hydrazine dehydrogenation to molecular nitrogen; however, on an Ir cluster, the ammonia undergoes successive dehydrogenation to form atomic nitrogen, followed by NN bond formation to N2. Moreover, rate of reaction constants, activation, and free energy calculations showed further evidence that production of N2 from its nitrogen atoms is sluggish and that the electro-catalyst may be considered as poisoned. Nonetheless, these calculations confirm that the onset potential for ammonia oxidation on iridium is lower than on platinum and this reaction starts at lower potential on Ir. In the presence of bimetallic catalysts, the iridium sites are more attractive for poisonous intermediates like NH and N, leaving platinum sites vacant for ammonia oxidation through hydrazine formation.
中文翻译:

铂和铱双金属簇上氨氧化动力学的理论方法
在Pt 3-x Ir x(x = 0-3)团簇上进行了密度泛函理论研究了氨气对氮的电氧化反应。在高斯09中研究了N 2 H 4-x(x = 0–3)的吸附以及簇组成对吸附的影响。发现N和NH是这些簇上最稳定的中间体,具有明显的稳定性。由铱的存在引起的。在Pt团簇上,氨的氧化机理涉及肼的形成,然后肼脱氢成分子氮。但是,在Ir团簇上,氨连续脱氢形成原子氮,然后与N 2形成N N键。此外,反应常数的速率,活化和自由能的计算显示了进一步的证据,表明由其氮原子产生的N 2缓慢并且该电催化剂可能被认为是有毒的。尽管如此,这些计算证实了铱上氨氧化的起始电势低于铂上,并且该反应从Ir上的低电势开始。在双金属催化剂的存在下,铱位点对于诸如NH和N之类的有毒中间体更具吸引力,而铂位点则空余地通过肼的形成而被氨氧化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号