Chemical Physics Letters ( IF 2.8 ) Pub Date : 2017-12-24 , DOI: 10.1016/j.cplett.2017.12.066 Wagner B. De Almeida , Patrick J. O'Malley
|
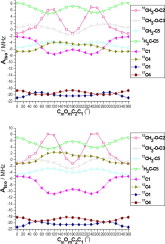
Ubiquinone is the key electron and proton transfer agent in biology. Its mechanism involves the formation of its intermediate one-electron reduced form, the ubisemiquinone radical. This is formed in a protein-bound form which permits the semiquinone to vary its electronic and redox properties. This can be achieved by hydrogen bonding acceptance by one or both oxygen atoms or as we now propose by restricted orientations for the methoxy groups of the headgroup. We show how the orientation of the two methoxy groups of the quinone headgroup affects the electronic structure of the semiquinone form and demonstrate a large dependence of the ubisemiquinone spin density distribution on the orientation each methoxy group takes with respect to the headgroup ring plane. This is shown to significantly modify associated hyperfine couplings which in turn needs to be accounted for in interpreting experimental values in vivo. The study uncovers the key potential role the methoxy group orientation can play in controlling the electronic structure and spin density of ubisemiquinone and provides an electronic-level insight into the variation in electron affinity and redox potential of ubiquinone as a function of the methoxy orientation. Taken together with the already known influence of cofactor conformation on heme and chlorophyll electronic structure, it reveals a more widespread role for cofactor conformational control of electronic structure and associated electron transfer in biology.
中文翻译:

自然中辅因子的构象控制:甲氧基的取向对泛半醌电子结构的影响。
泛醌是生物学中的关键电子和质子转移剂。其机理涉及其中间单电子还原形式泛半醌自由基的形成。它以蛋白质结合形式形成,允许半醌改变其电子和氧化还原特性。这可以通过一个或两个氧原子的氢键键合来实现,或者如我们现在所建议的那样,通过限制头基的甲氧基的取向来实现。我们展示了醌头基的两个甲氧基的取向如何影响半醌形式的电子结构,并证明了泛半醌自旋密度分布对每个甲氧基相对于头基环平面的取向有很大的依赖性。已显示这显着修饰了相关的超精细偶联,这反过来又需要在解释体内实验值时加以考虑。这项研究揭示了甲氧基的取向在控制泛半醌的电子结构和自旋密度方面可以发挥的关键潜在作用,并提供了电子水平的洞察力,以了解泛醌的电子亲和力和氧化还原电位随甲氧基取向的变化。连同已知的辅因子构象对血红素和叶绿素电子结构的影响,它揭示了在生物学中辅助因子构象控制电子结构和相关电子转移的作用更加广泛。这项研究揭示了甲氧基的取向在控制泛半醌的电子结构和自旋密度方面可以发挥的关键潜在作用,并提供了电子水平的洞察力,以了解泛醌的电子亲和力和氧化还原电位随甲氧基取向的变化。连同已知的辅因子构象对血红素和叶绿素电子结构的影响,它揭示了在生物学中辅助因子构象控制电子结构和相关电子转移的作用更加广泛。这项研究揭示了甲氧基的取向在控制泛半醌的电子结构和自旋密度方面可以发挥的关键潜在作用,并提供了电子水平的洞察力,以了解泛醌的电子亲和力和氧化还原电位随甲氧基取向的变化。连同已知的辅因子构象对血红素和叶绿素电子结构的影响,它揭示了在生物学中辅助因子构象控制电子结构和相关电子转移的作用更加广泛。











































 京公网安备 11010802027423号
京公网安备 11010802027423号