Chemical Physics Letters ( IF 2.8 ) Pub Date : 2017-12-24 , DOI: 10.1016/j.cplett.2017.12.041 Santanu Roy , Mirza Galib , Gregory K. Schenter , Christopher J. Mundy
|
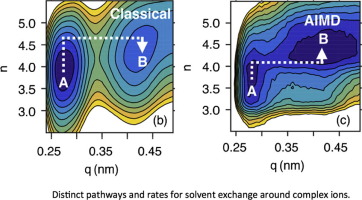
The phenomena of solvent exchange control the process of solvating ions, protons, and charged molecules. Building upon our extension of Marcus’ philosophy of electron transfer, we provide a new perspective of ultrafast solvent exchange mechanism around ions measurable by two-dimensional infrared (2DIR) spectroscopy. In this theory, solvent rearrangement drives an ion-bound water to an activated state of higher coordination number, triggering ion-water separation that leads to the solvent-bound state of the water molecule. This ion-bound to solvent-bound transition rate for a -water system is computed using ab initio molecular dynamics and Marcus theory, and is found to be in excellent agreement with the 2DIR measurement.
中文翻译:

马库斯理论与溶剂动力学超快光谱的关系
溶剂交换现象控制着离子,质子和带电分子的溶剂化过程。在扩展了马库斯(Marcus)电子转移哲学的基础上,我们提供了围绕离子的超快速溶剂交换机制的新观点,该离子可通过二维红外(2DIR)光谱测量。在此理论中,溶剂重排将离子结合的水驱动到更高配位数的活化状态,从而触发离子水分离,从而导致水分子与溶剂结合的状态。该离子结合到溶剂结合的转变速率为-水系统是使用从头算分子动力学和Marcus理论计算得出的,并且与2DIR测量非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号