Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2017-12-25 , DOI: 10.1016/j.jhazmat.2017.12.062 Haijun Chen , Zhe Chen , Guixia Zhao , Zhibin Zhang , Chao Xu , Yunhai Liu , Jing Chen , Li Zhuang , Tasawar Haya , Xiangke Wang
|
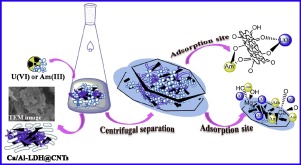
Ca/Al layered double hydroxide decorated carbon nanotube (Ca/Al-LDH@CNTs) composites were fabricated by co-precipitation method and hydrothermal aged treatment. The prepared Ca/Al-LDH@CNTs was characterized by SEM, TEM, EDS, XRD, FT-IR, UV-Vis and XPS techniques, and applied to remove U(VI) from aqueous solutions under various environmental conditions (i.e., pH, ionic strength, temperature and contact time). The results indicated that the adsorption of U(VI) on Ca/Al-LDH@CNTs was four times higher than that of U(VI) on bare CNTs. The kinetic investigations reflected the chemisorption of U(VI) on Ca/Al-LDH@CNTs through oxygen-containing functional groups. The adsorption isotherms demonstrated that the adsorption of U(VI) was well fitted by Langmuir model and the maximum adsorption capacity of U(VI) on Ca/Al-LDH@CNTs was calculated to be 382.9 mg·g-1 at 289.15 K. The thermodynamic parameters calculated from temperature-dependent isotherms suggested that U(VI) adsorption on Ca/Al-LDH@CNTs were endothermic and spontaneous process. Furthermore, Ca/Al-LDH@CNTs could remove ~91% of 241Am(III) at pH= 8.0, which confirmed Ca/Al-LDH@CNTs as a promising material for multiply low level radionuclides’ pollution remediation.
中文翻译:

Ca / Al层状双氢氧化物@碳纳米管复合材料增强废水中U(VI)和241 Am(III)的吸附
采用共沉淀法和水热时效法制备了Ca / Al层状双氢氧化物修饰的碳纳米管(Ca / Al-LDH @ CNTs)复合材料。通过SEM,TEM,EDS,XRD,FT-IR,UV-Vis和XPS技术对制备的Ca / Al-LDH @ CNTs进行表征,并用于在各种环境条件下(即pH值)从水溶液中去除U(VI)。 ,离子强度,温度和接触时间)。结果表明,U(VI)在Ca / Al-LDH @ CNTs上的吸附量是U(VI)在裸露的CNTs上的吸附量的四倍。动力学研究反映了U(VI)通过含氧官能团在Ca / Al-LDH @ CNTs上的化学吸附。吸附等温线表明,Langmuir模型很好地拟合了U(VI)的吸附,在Ca / Al-LDH @ CNTs上U(VI)的最大吸附容量为382.9 mg·g-1在289.15K。根据温度等温线计算的热力学参数表明,U(VI)在Ca / Al-LDH @ CNTs上的吸附是吸热和自发过程。此外,Ca / Al-LDH @ CNTs可以在pH = 8.0时去除241 Am(III)中的约91%,这证实了Ca / Al-LDH @ CNTs是用于低水平放射性核素污染修复的有前途的材料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号