当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of tunable, red fluorescent aggregation-enhanced emissive organic fluorophores: stimuli-responsive high contrast off–on fluorescence switching†
CrystEngComm ( IF 2.6 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1039/c7ce01867c Palamarneri Sivaraman Hariharan 1, 2, 3, 4, 5 , Parthasarathy Gayathri 1, 2, 3, 4, 5 , Anu Kundu 1, 2, 3, 4, 5 , Subramanian Karthikeyan 1, 5, 6, 7 , Dohyun Moon 8, 9, 10, 11 , Savarimuthu Philip Anthony 1, 2, 3, 4, 5
CrystEngComm ( IF 2.6 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1039/c7ce01867c Palamarneri Sivaraman Hariharan 1, 2, 3, 4, 5 , Parthasarathy Gayathri 1, 2, 3, 4, 5 , Anu Kundu 1, 2, 3, 4, 5 , Subramanian Karthikeyan 1, 5, 6, 7 , Dohyun Moon 8, 9, 10, 11 , Savarimuthu Philip Anthony 1, 2, 3, 4, 5
Affiliation
|
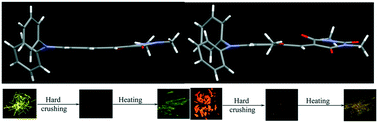
Triphenylamine (TPA) and N-methylbarbituric acid/indanedione-based donor–acceptor derivatives were synthesized and demonstrated molecular conformation and packing dependent tunable fluorescence from yellow to red in the solid state. TPA with N-methylbarbituric acid (BA-1) showed bright yellow fluorescence (λmax = 550 nm, Φf = 22.8%) whereas OCH3 substitution at the phenyl rings of TPA, (BA-2 and BA-3) produced strong red (λmax = 602 nm, Φf = 41.1%) and orange fluorescent solids (λmax = 582 nm, Φf = 19.1%). Indanedione acceptor dyes exhibited red to deep red fluorescence. ID-1 showed red fluorescence at 604 nm (Φf = 17.7%) whereas ID-2 and ID-3 showed fluorescence at 611 (Φf = 19.4%) and 636 nm (Φf = 14.1%) in the solid state. Solid state structural analysis revealed alteration of the molecular conformation and packing by OCH3 substitution which led to tunable fluorescence. BA and ID compounds showed turn-off fluorescence/substantially reduced fluorescence intensity upon hard crushing (Φf = 1.2 to 3.1%). Interestingly, heating of BA and ID crushed powders led to turn-on fluorescence/significant enhancement of fluorescence intensity (Φf = 5.6 to 22.5%). Powder X-ray diffraction (PXRD) studies indicated the conversion of the crystalline phase to amorphous and the amorphous phase to crystalline by hard crushing and heating. The reversible conversion of the crystalline phase to amorphous phase was responsible for the fluorescence switching of BA and ID. Computational studies have been performed to get an insight into the energy level modulation upon the change of the molecular conformation. Thus, we have presented the facile preparation of strong red fluorescent dyes exhibiting high contrast stimuli-responsive reversible dark and bright fluorescence switching in the solid state.
中文翻译:

可调的红色荧光团聚增强的发射有机荧光团的合成:刺激响应的高对比度关闭-开启荧光切换†
合成了三苯胺(TPA)和基于N-甲基巴比妥酸/茚满二酮的供体-受体衍生物,并证明了分子构象和固体状态下从黄色到红色的依赖包装的可调荧光。TPA与Ñ -methylbarbituric酸(BA- 1)显示出明亮的黄色荧光(λ最大= 550纳米,Φ ˚F = 22.8%),而OCH 3在TPA的苯环取代,(BA- 2和BA- 3)产生的强红色(λ最大= 602纳米,Φ ˚F = 41.1%)和橙色荧光的固体(λ最大= 582纳米,Φ ˚F = 19.1%)。茚满二酮受体染料显示红色至深红色荧光。ID- 1显示出红色荧光在604纳米(Φ ˚F = 17.7%),而ID- 2和ID- 3在611(显示荧光Φ ˚F = 19.4%)和636纳米(Φ ˚F在固态= 14.1%)。固态结构分析表明,OCH 3取代会改变分子构象和堆积,从而导致可调荧光。BA和ID化合物在硬粉碎后显示出关闭的荧光/荧光强度大大降低了(Φ ˚F = 1.2〜3.1%)。有趣的是,BA和ID粉碎粉末的加热导致接通荧光/荧光强度的增强显著(Φ ˚F= 5.6至22.5%)。粉末X射线衍射(PXRD)研究表明,通过硬粉碎和加热,结晶相转化为非晶态,而非晶相转化为结晶。结晶相向非晶相的可逆转化是BA和ID荧光转换的原因。已经进行了计算研究,以了解分子构象变化时的能级调节。因此,我们提出了在固体状态下显示高对比度刺激响应可逆的暗和亮荧光转换的强红色荧光染料的简便制备方法。
更新日期:2017-12-26
中文翻译:

可调的红色荧光团聚增强的发射有机荧光团的合成:刺激响应的高对比度关闭-开启荧光切换†
合成了三苯胺(TPA)和基于N-甲基巴比妥酸/茚满二酮的供体-受体衍生物,并证明了分子构象和固体状态下从黄色到红色的依赖包装的可调荧光。TPA与Ñ -methylbarbituric酸(BA- 1)显示出明亮的黄色荧光(λ最大= 550纳米,Φ ˚F = 22.8%),而OCH 3在TPA的苯环取代,(BA- 2和BA- 3)产生的强红色(λ最大= 602纳米,Φ ˚F = 41.1%)和橙色荧光的固体(λ最大= 582纳米,Φ ˚F = 19.1%)。茚满二酮受体染料显示红色至深红色荧光。ID- 1显示出红色荧光在604纳米(Φ ˚F = 17.7%),而ID- 2和ID- 3在611(显示荧光Φ ˚F = 19.4%)和636纳米(Φ ˚F在固态= 14.1%)。固态结构分析表明,OCH 3取代会改变分子构象和堆积,从而导致可调荧光。BA和ID化合物在硬粉碎后显示出关闭的荧光/荧光强度大大降低了(Φ ˚F = 1.2〜3.1%)。有趣的是,BA和ID粉碎粉末的加热导致接通荧光/荧光强度的增强显著(Φ ˚F= 5.6至22.5%)。粉末X射线衍射(PXRD)研究表明,通过硬粉碎和加热,结晶相转化为非晶态,而非晶相转化为结晶。结晶相向非晶相的可逆转化是BA和ID荧光转换的原因。已经进行了计算研究,以了解分子构象变化时的能级调节。因此,我们提出了在固体状态下显示高对比度刺激响应可逆的暗和亮荧光转换的强红色荧光染料的简便制备方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号