Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2017-12-23 , DOI: 10.1016/j.jinorgbio.2017.12.014 Hiroaki Isago , Harumi Fujita , Tamotsu Sugimori
|
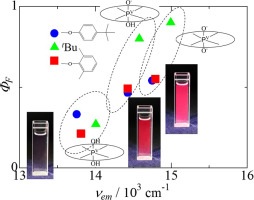
Spectral (optical absorption and emission) properties of three amphoteric phosphorous(V)-phthalocyanine derivatives, [P(Pc)(O)OH], where Pc = tetra(tert-butyl)phthalocyaninate (tbpc), tetrakis(2′,6′-dimethylphenoxy)phthalocyaninate (tppc), and octakis(4′-tert-butylphenoxy)phthalocyaninate (obppc), have been investigated in ethanolic solutions. Spectral changes upon protonation/deprotonation (the reaction sites have been determined to be their axial ligands by magnetic circular dichroism study) are drastic and rapid. All the initial ([P(Pc)(O)OH]), protonated ([P(Pc)(OH)2]+), and deprotonated ([P(Pc)(O)2]−) species are possessed with sufficient brightness (defined as the product of their molar extinction coefficient, ε (in M− 1 cm− 1), and fluorescence quantum yield, ΦF) in bio-imaging window (650–900 nm). For example, spectral characteristics of the tbpc derivatives have been determined as follows: ε = 1.65 × 105 (absorption maximum 676 nm) and ΦF = 0.80 (emission maximum 686 nm) for [P(tbpc)(O)(OH)] while ε = 1.45 × 105 (697 nm) and ΦF = 0.27 (714 nm) for [P(tbpc)(OH)2]+, and ε = 2.25 × 105 (662 nm) and ΦF = 0.90 (667 nm) for [P(tbpc)(O)2]−. Emission of tppc and obppc derivatives behave in essentially the same manner irrespective of nature of the peripheral substituents and hence ΦF values are greater with increasing emission peak wavenumbers in line with the “energy gap law”. These characteristics make these compounds promising candidates as chemical probes for deep-tissue bio-imaging.
中文翻译:

两性磷(V)-酞菁类化合物作为质子驱动的可转换荧光剂,用于深部组织生物成像
三种两性磷(V)-酞菁衍生物[P(Pc)(O)OH]的光谱(光学吸收和发射)性质,其中Pc =酞菁四(叔丁基)酞菁(tbpc),四(2',6已经在乙醇溶液中研究了'-二甲基苯氧基)酞菁盐(tppc)和辛基(4'-叔丁基苯氧基)酞菁盐(obppc)。质子化/去质子化时的光谱变化是剧烈且快速的(反应位点已通过磁性圆二色性研究确定为它们的轴向配体)。所有初始([P(Pc)(O)OH]),质子化([P(Pc)(OH)2 ] +)和去质子化([P(Pc)(O)2 ] -)物种具有以足够的亮度(被定义为它们的摩尔消光系数,ε(在M的产物- 1 厘米1 - ),和荧光量子产率,Φ ˚F在生物成像窗口)(650-900纳米)。例如,TBPC衍生物的光谱特性已经被确定为如下:ε= 1.65×10 5(最大吸收676纳米)和Φ ˚F = 0.80(最大发射686纳米)为[P(TBPC)(O)(OH) ],而ε= 1.45×10 5(697纳米)和Φ ˚F = 0.27(714纳米)为[P(TBPC)(OH)2 ] +,和ε= 2.25×10 5(662纳米)和Φ ˚F = 0.90对于[P(tbpc)(O)2为(667 nm)] -。TPPC和obppc衍生物的发射表现在基本相同的方式而不管周围取代基,因此Φ的性质的˚F值是较大值与“能隙法”在线增加发射峰的波数。这些特性使这些化合物有望成为深组织生物成像的化学探针。











































 京公网安备 11010802027423号
京公网安备 11010802027423号