当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Structure-Based Assembly Screen of Protein Cage Libraries in Living Cells: Experimentally Repacking a Protein–Protein Interface To Recover Cage Formation in an Assembly-Frustrated Mutant
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.biochem.7b01000 Thomas A. Cornell 1, 2 , Maziar S. Ardejani 1, 2 , Jing Fu 2 , Stephanie H. Newland 3 , Yu Zhang 2 , Brendan P. Orner 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.biochem.7b01000 Thomas A. Cornell 1, 2 , Maziar S. Ardejani 1, 2 , Jing Fu 2 , Stephanie H. Newland 3 , Yu Zhang 2 , Brendan P. Orner 1, 2
Affiliation
|
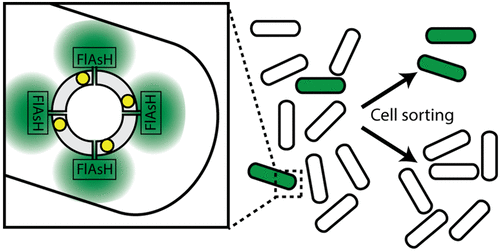
Cage proteins, which assemble into often highly symmetric hollow nanoscale capsules, have great potential in applications as far reaching as drug delivery, hybrid nanomaterial engineering, and catalysis. In addition, they are promising model systems for understanding how cellular nanostructures are constructed through protein–protein interactions, and they are beginning to be used as scaffolds for synthetic biology approaches. Recently, there has been renewed interest in the engineering of protein cages, and in support of these strategies, we have recently described a fluorescence-based assay for protein cage assembly that is specific for certain oligomerization states and symmetry-related protein–protein interfaces. In this work, we expand this assay to living cells and a high-throughput assay for screening protein cage libraries using flow cytometry. As a proof of principle, we apply this technique to the screening of libraries of a double-alanine mutant of the mini-ferritin, DNA-binding protein from starved cells (Dps). This mutant, due to disruption of key protein–protein interactions, is unable to assemble into a cage. Randomization of residues surrounding the double mutation afforded a repacked interface and proteins with recovered cage formation, demonstrating the strength and utility of this approach.
中文翻译:

活细胞中蛋白质笼子库的基于结构的组装屏幕:实验性地重新包装蛋白质-蛋白质界面以回收受挫的突变体中的笼子形成。
笼状蛋白通常组装成高度对称的中空纳米级胶囊,在药物输送,杂化纳米材料工程和催化等领域具有广阔的应用前景。此外,它们是有前途的模型系统,可用于理解如何通过蛋白质-蛋白质相互作用构建细胞纳米结构,并且它们已开始用作合成生物学方法的支架。最近,人们对蛋白笼的工程技术有了新的兴趣,为了支持这些策略,我们最近描述了一种基于荧光的蛋白笼装配检测方法,该方法对某些寡聚化状态和与对称性相关的蛋白-蛋白界面具有特异性。在这项工作中,我们将这种测定法扩展到活细胞,并通过流式细胞术筛选蛋白笼文库的高通量测定法。作为原理的证明,我们将此技术应用于从饥饿细胞(Dps)筛选迷你铁蛋白,DNA结合蛋白的双丙氨酸突变体的文库。该突变体由于关键的蛋白质-蛋白质相互作用被破坏,因此无法组装成笼子。围绕双突变的残基的随机化提供了重新包装的界面和具有恢复的笼形成的蛋白质,证明了该方法的强度和实用性。无法组装到笼子里。围绕双突变的残基的随机化提供了重新包装的界面和具有恢复的笼形成的蛋白质,证明了该方法的强度和实用性。无法组装到笼子里。围绕双突变的残基的随机化提供了重新包装的界面和具有恢复的笼形成的蛋白质,证明了该方法的强度和实用性。
更新日期:2018-01-17
中文翻译:

活细胞中蛋白质笼子库的基于结构的组装屏幕:实验性地重新包装蛋白质-蛋白质界面以回收受挫的突变体中的笼子形成。
笼状蛋白通常组装成高度对称的中空纳米级胶囊,在药物输送,杂化纳米材料工程和催化等领域具有广阔的应用前景。此外,它们是有前途的模型系统,可用于理解如何通过蛋白质-蛋白质相互作用构建细胞纳米结构,并且它们已开始用作合成生物学方法的支架。最近,人们对蛋白笼的工程技术有了新的兴趣,为了支持这些策略,我们最近描述了一种基于荧光的蛋白笼装配检测方法,该方法对某些寡聚化状态和与对称性相关的蛋白-蛋白界面具有特异性。在这项工作中,我们将这种测定法扩展到活细胞,并通过流式细胞术筛选蛋白笼文库的高通量测定法。作为原理的证明,我们将此技术应用于从饥饿细胞(Dps)筛选迷你铁蛋白,DNA结合蛋白的双丙氨酸突变体的文库。该突变体由于关键的蛋白质-蛋白质相互作用被破坏,因此无法组装成笼子。围绕双突变的残基的随机化提供了重新包装的界面和具有恢复的笼形成的蛋白质,证明了该方法的强度和实用性。无法组装到笼子里。围绕双突变的残基的随机化提供了重新包装的界面和具有恢复的笼形成的蛋白质,证明了该方法的强度和实用性。无法组装到笼子里。围绕双突变的残基的随机化提供了重新包装的界面和具有恢复的笼形成的蛋白质,证明了该方法的强度和实用性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号