当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption of Kinetic Hydrate Inhibitors on Growing Surfaces: A Molecular Dynamics Study
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1021/acs.jpcb.7b10356 Takuma Yagasaki 1 , Masakazu Matsumoto 1 , Hideki Tanaka 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1021/acs.jpcb.7b10356 Takuma Yagasaki 1 , Masakazu Matsumoto 1 , Hideki Tanaka 1
Affiliation
|
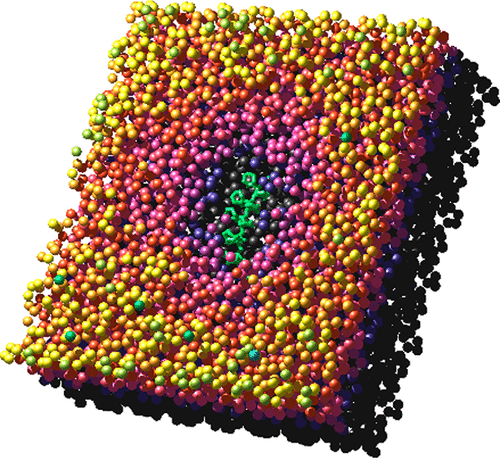
We investigate the mechanism of a typical kinetic hydrate inhibitor (KHI), polyvinylcaprolactam (PVCap), which has been applied to prevent hydrate plugs from forming in gas pipe lines, using molecular dynamics simulations of crystal growth of ethylene oxide hydrate. Water-soluble ethylene oxide is chosen as a guest species to avoid problems associated with the presence of the gas phase in the simulation cell such as slow crystal growth. A PVCap dodecamer adsorbs irreversibly on the hydrate surface which grows at supercooling of 3 K when the hydrophobic part of two pendent groups are trapped in open cages at the surface. The amide hydrogen bonds make no contribution to the adsorption. PVCap can adsorb on various crystallographic planes of sI hydrate. This is in contrast to antifreeze proteins, each of which prefers a specific plane of ice. The trapped PVCap gives rise to necessarily the concave surface of the hydrate. The crystal growth rate decreases with increasing surface curvature, indicating that the inhibition by PVCap is explained by the Gibbs–Thomson effect.
中文翻译:

动力学水合物抑制剂在生长表面的吸附:分子动力学研究
我们使用环氧乙烷水合物晶体生长的分子动力学模拟,研究了典型的动力学水合物抑制剂(KHI),聚乙烯基己内酰胺(PVCap)的机理,该机理已用于防止天然气管道中形成水合物塞。选择水溶性环氧乙烷作为客体,以避免与模拟单元中气相的存在相关的问题,例如晶体生长缓慢。当两个悬垂基团的疏水部分被困在表面开放的笼子中时,PVCap十二聚体不可逆地吸附在水合物表面上,该水合物表面在3 K过冷时生长。酰胺氢键对吸附没有贡献。PVCap可以吸附在sI水合物的各种晶体平面上。这与防冻蛋白相反,后者每个都喜欢特定的冰平面。捕集的PVCap必定会引起水合物的凹面。晶体的生长速率随着表面曲率的增加而降低,表明PVCap的抑制作用是由吉布斯-汤姆森效应解释的。
更新日期:2017-12-26
中文翻译:

动力学水合物抑制剂在生长表面的吸附:分子动力学研究
我们使用环氧乙烷水合物晶体生长的分子动力学模拟,研究了典型的动力学水合物抑制剂(KHI),聚乙烯基己内酰胺(PVCap)的机理,该机理已用于防止天然气管道中形成水合物塞。选择水溶性环氧乙烷作为客体,以避免与模拟单元中气相的存在相关的问题,例如晶体生长缓慢。当两个悬垂基团的疏水部分被困在表面开放的笼子中时,PVCap十二聚体不可逆地吸附在水合物表面上,该水合物表面在3 K过冷时生长。酰胺氢键对吸附没有贡献。PVCap可以吸附在sI水合物的各种晶体平面上。这与防冻蛋白相反,后者每个都喜欢特定的冰平面。捕集的PVCap必定会引起水合物的凹面。晶体的生长速率随着表面曲率的增加而降低,表明PVCap的抑制作用是由吉布斯-汤姆森效应解释的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号