当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A universal method for the determination of polysorbate 80 in monoclonal antibodies and novel protein therapeutic formulations†
Analytical Methods ( IF 2.7 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1039/c7ay02537h Veerendra Koppolu 1, 2, 3, 4, 5 , Bhargavi Vemulapalli 1, 2, 3, 4, 5 , Jason Thomas 1, 2, 3, 4, 5 , Sheau-Chiann Wang 1, 2, 3, 4, 5 , Jon Borman 1, 2, 3, 4, 5
Analytical Methods ( IF 2.7 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1039/c7ay02537h Veerendra Koppolu 1, 2, 3, 4, 5 , Bhargavi Vemulapalli 1, 2, 3, 4, 5 , Jason Thomas 1, 2, 3, 4, 5 , Sheau-Chiann Wang 1, 2, 3, 4, 5 , Jon Borman 1, 2, 3, 4, 5
Affiliation
|
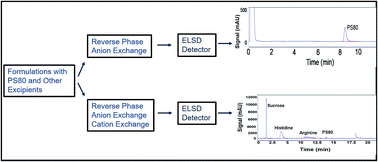
An evaporative light scattering detector (ELSD) based HPLC method that requires minimal sample preparation has been developed to quantitate polysorbate 80 (PS80) in therapeutic proteins. The method utilizes a mixed-mode chromatography column and a step gradient of formic acid and acetonitrile to separate PS80 from proteins and formulation excipients. Proteins and hydrophilic formulation excipients are not retained in the column and are eluted in the void volume. The retained hydrophobic, heterogenous PS80 species can be quantified as a single peak using the ELSD. A universal standard curve for PS80 in HPLC grade water was utilized in this method. The method accurately quantified PS80 in 18 different sample formulations including monoclonal antibodies, bispecific antibodies, antibody drug conjugates (ADCs), and bispecific ADCs, thereby establishing a truly selective method free of matrix interference. The assay performance evaluated for precision, specificity, accuracy, and linearity demonstrated the method qualification in the range of 0.05–0.6 mg ml−1 PS80 concentrations and up to 150 mg ml−1 protein concentration. The method also accurately monitored the reduction of PS80 in samples subjected to thermal and long-term storage stresses. The methodology was found to be applicable for the quantitation of polymers PLGA and Pluronic F127. The method was further modified to create a 2-dimensional liquid chromatography ELSD system and quantitated other formulation excipients histidine, arginine, and sucrose in the presence of PS80.
中文翻译:

测定单克隆抗体和新型蛋白质治疗剂中聚山梨酯80的通用方法†
已经开发了一种基于蒸发光散射检测器(ELSD)的HPLC方法,只需最少的样品制备即可定量治疗性蛋白质中的聚山梨酯80(PS80)。该方法利用混合模式色谱柱和甲酸和乙腈的逐步梯度从蛋白质和制剂赋形剂中分离出PS80。蛋白质和亲水性制剂赋形剂未保留在色谱柱中,并在空隙体积中洗脱。可以使用ELSD将保留的疏水性,异质PS80物种定量为单个峰。该方法使用了HPLC级水中PS80的通用标准曲线。该方法可准确定量18种不同样品制剂中的PS80,包括单克隆抗体,双特异性抗体,抗体药物偶联物(ADC)和双特异性ADC,从而建立了一种没有矩阵干扰的真正的选择性方法。评估的精密度,特异性,准确性和线性度的分析性能证明了方法的合格性在0.05–0.6 mg ml的范围内-1 PS80浓度和最高150 mg ml -1蛋白质浓度。该方法还可以准确地监测遭受热应力和长期存储应力的样品中PS80的降低。发现该方法可用于定量聚合物PLGA和Pluronic F127。对该方法进行了进一步修改,以创建二维液相色谱ELSD系统,并在PS80存在的情况下对其他制剂赋形剂组氨酸,精氨酸和蔗糖进行了定量。
更新日期:2017-12-26
中文翻译:

测定单克隆抗体和新型蛋白质治疗剂中聚山梨酯80的通用方法†
已经开发了一种基于蒸发光散射检测器(ELSD)的HPLC方法,只需最少的样品制备即可定量治疗性蛋白质中的聚山梨酯80(PS80)。该方法利用混合模式色谱柱和甲酸和乙腈的逐步梯度从蛋白质和制剂赋形剂中分离出PS80。蛋白质和亲水性制剂赋形剂未保留在色谱柱中,并在空隙体积中洗脱。可以使用ELSD将保留的疏水性,异质PS80物种定量为单个峰。该方法使用了HPLC级水中PS80的通用标准曲线。该方法可准确定量18种不同样品制剂中的PS80,包括单克隆抗体,双特异性抗体,抗体药物偶联物(ADC)和双特异性ADC,从而建立了一种没有矩阵干扰的真正的选择性方法。评估的精密度,特异性,准确性和线性度的分析性能证明了方法的合格性在0.05–0.6 mg ml的范围内-1 PS80浓度和最高150 mg ml -1蛋白质浓度。该方法还可以准确地监测遭受热应力和长期存储应力的样品中PS80的降低。发现该方法可用于定量聚合物PLGA和Pluronic F127。对该方法进行了进一步修改,以创建二维液相色谱ELSD系统,并在PS80存在的情况下对其他制剂赋形剂组氨酸,精氨酸和蔗糖进行了定量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号