当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modeling the response of a control-released ion-selective electrode and employing it for the study of permanganate oxidation kinetics†
Analytical Methods ( IF 3.1 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1039/c7ay02735d Dean Song 1, 2, 3, 4, 5 , Rongning Liang 6, 7, 8, 9, 10 , Xiaohua Jiang 6, 7, 8, 9, 10 , Huiqing Sun 1, 2, 3, 4, 5 , Fanyu Kong 1, 2, 3, 4, 5 , Bo Lv 1, 2, 3, 4, 5 , Qiannan Fang 1, 2, 3, 4, 5 , Wei Qin 6, 7, 8, 9, 10
Analytical Methods ( IF 3.1 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1039/c7ay02735d Dean Song 1, 2, 3, 4, 5 , Rongning Liang 6, 7, 8, 9, 10 , Xiaohua Jiang 6, 7, 8, 9, 10 , Huiqing Sun 1, 2, 3, 4, 5 , Fanyu Kong 1, 2, 3, 4, 5 , Bo Lv 1, 2, 3, 4, 5 , Qiannan Fang 1, 2, 3, 4, 5 , Wei Qin 6, 7, 8, 9, 10
Affiliation
|
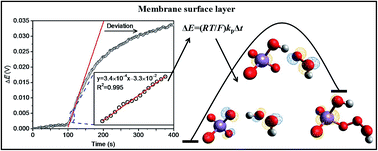
Although polymeric membrane ion-selective electrodes (ISEs) based on outward ion fluxes have been found analytically useful, there is still a lack of a theoretical framework for this detection system. In this study, we attempted to model the response of this kind of permanganate ISE and employed this ISE to analyze the rapid MnO4−/H2O2 reaction. This response is attributed to H2O2 oxidation with MnO4− that is released from the inner solution to the membrane surface layer. The results show that the experimental data can be fitted well to the proposed model that is elucidated mathematically from the viewpoint of chemical kinetics. The second-order rate constant is determined at a near neutral pH and is in agreement with the acid dissociation law to provide the specific value of 370 M−1 s−1. The kinetic mechanism was then investigated by performing DFT calculations. Via analysis of the Mn–O bond length and the HOMO orbital, it has been found that the studied redox system functions similarly as the so-called hydrogen abstraction mechanism with an energy barrier of 24.5 kcal mol−1. This study is considered to be the first report on the simulation of MnO4− attack at the O–H bond. On the basis of the transition state theory and previous studies on MnO4− attack at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H bonds, the relationship between the experimental rate constant and computational energy barrier is finally constructed. The result indicates the validity of our proposed method and makes the control-released ISE a very promising platform to study the kinetics.
C and C–H bonds, the relationship between the experimental rate constant and computational energy barrier is finally constructed. The result indicates the validity of our proposed method and makes the control-released ISE a very promising platform to study the kinetics.
中文翻译:

模拟控制释放的离子选择电极的响应并将其用于研究高锰酸盐的氧化动力学†
尽管已经发现基于向外的离子通量的聚合物膜离子选择电极(ISE)在分析上是有用的,但仍缺乏该检测系统的理论框架。在这项研究中,我们试图这种高锰酸盐ISE的的响应进行建模和采用这种ISE分析快速的MnO 4 - / H 2 ö 2反应。这种反应是由于与H 2 ö 2氧化的MnO与4 -它从内部溶液释放到膜表层。结果表明,实验数据可以很好地拟合所提出的模型,该模型从化学动力学的角度进行了数学解释。二阶速率常数在接近中性的pH值下确定,并且与酸解离定律一致,可提供370 M -1 s -1的比值。然后通过执行DFT计算研究动力学机理。通过对Mn–O键长和HOMO轨道的分析,发现所研究的氧化还原系统的功能类似于所谓的氢提取机理,其能垒为24.5 kcal mol -1。这项研究被认为是对的MnO模拟的第一次报告4 -攻击时将O-H键。在过渡态理论和以往的研究上的MnO的基础上4 -在C攻击![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C和C-H键,实验速率常数和计算能量势垒之间的关系最终构造。结果表明我们提出的方法的有效性,并使控制释放的ISE成为研究动力学的非常有前途的平台。
C和C-H键,实验速率常数和计算能量势垒之间的关系最终构造。结果表明我们提出的方法的有效性,并使控制释放的ISE成为研究动力学的非常有前途的平台。
更新日期:2017-12-26
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–H bonds, the relationship between the experimental rate constant and computational energy barrier is finally constructed. The result indicates the validity of our proposed method and makes the control-released ISE a very promising platform to study the kinetics.
C and C–H bonds, the relationship between the experimental rate constant and computational energy barrier is finally constructed. The result indicates the validity of our proposed method and makes the control-released ISE a very promising platform to study the kinetics.
中文翻译:

模拟控制释放的离子选择电极的响应并将其用于研究高锰酸盐的氧化动力学†
尽管已经发现基于向外的离子通量的聚合物膜离子选择电极(ISE)在分析上是有用的,但仍缺乏该检测系统的理论框架。在这项研究中,我们试图这种高锰酸盐ISE的的响应进行建模和采用这种ISE分析快速的MnO 4 - / H 2 ö 2反应。这种反应是由于与H 2 ö 2氧化的MnO与4 -它从内部溶液释放到膜表层。结果表明,实验数据可以很好地拟合所提出的模型,该模型从化学动力学的角度进行了数学解释。二阶速率常数在接近中性的pH值下确定,并且与酸解离定律一致,可提供370 M -1 s -1的比值。然后通过执行DFT计算研究动力学机理。通过对Mn–O键长和HOMO轨道的分析,发现所研究的氧化还原系统的功能类似于所谓的氢提取机理,其能垒为24.5 kcal mol -1。这项研究被认为是对的MnO模拟的第一次报告4 -攻击时将O-H键。在过渡态理论和以往的研究上的MnO的基础上4 -在C攻击
![[双键,长度为m-破折号]](https://www.rsc.org/images/entities/char_e001.gif) C和C-H键,实验速率常数和计算能量势垒之间的关系最终构造。结果表明我们提出的方法的有效性,并使控制释放的ISE成为研究动力学的非常有前途的平台。
C和C-H键,实验速率常数和计算能量势垒之间的关系最终构造。结果表明我们提出的方法的有效性,并使控制释放的ISE成为研究动力学的非常有前途的平台。



























 京公网安备 11010802027423号
京公网安备 11010802027423号