当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extraction of Citric Acid and Maleic Acid from Their Aqueous Solutions Using a Phosphorus-Bonded Extractant, Tri-n-octylphosphineoxide, and a Secondary Amine, Dioctylamine
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1021/acs.jced.7b00562 Erdem Hasret 1 , Şah İsmail Kırbaşlar 1 , Hasan Uslu 2, 3
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2017-12-26 00:00:00 , DOI: 10.1021/acs.jced.7b00562 Erdem Hasret 1 , Şah İsmail Kırbaşlar 1 , Hasan Uslu 2, 3
Affiliation
|
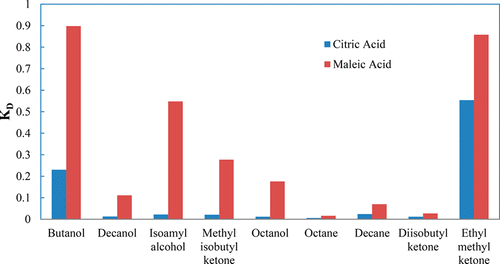
The aim of this study is to investigate the extraction of citric acid and maleic acid from their aqueous solutions using different solvent–extractant mixtures in order to find the most effective composition for the extraction. Citric and maleic acids are chosen as the carboxylic acids due to their commercial worth in the industrial processes. Carboxylic acids are recovered from their aqueous solutions by reactive extraction, a promising liquid–liquid extraction technique, using trioctylphosphineoxide (TOPO), and a secondary amine called dioctylamine (DOA). TOPO has been chosen as an extractant since it has low water solubility, high stability, and it is more environmentally friendly than the amine-type extractants. For other amine extractants used in reactive extraction processes, there is a lack of study in the literature about the extraction of these acids with DOA. Extractants were dissolved in nine different solvents (butanol, decanol, octanol, isoamylalcohol, octane, decane, methylisobutylketone, and diisobutylketone) having different chemical structures. All experiments were carried out at 298.15 K. Comparisons of the results were made using the distribution coefficient (D), loading factor (Z), and the extraction yield (%E). It has been observed that a considerable amount of citric acid and maleic acid extraction was achieved using DOA (in the range of 13.95–99.19% and 31.94–99.41% according to the diluent used for citric acid and maleic acid, respectively) compared to that achieved with TOPO (in the range of 0.81–67.12% and 16.25–89.33% according to the diluent used for citric acid and maleic acid, respectively).
中文翻译:

使用磷键合萃取剂三正辛基膦氧化物和仲胺二辛胺从水溶液中萃取柠檬酸和马来酸
这项研究的目的是研究使用不同的溶剂萃取剂混合物从其水溶液中萃取柠檬酸和马来酸,以便找到最有效的萃取成分。选择柠檬酸和马来酸作为羧酸,因为它们在工业过程中具有商业价值。通过反应萃取,一种有前途的液-液萃取技术,使用三辛基氧化膦(TOPO)和称为二辛胺(DOA)的仲胺,可以从水溶液中回收羧酸。TOPO已被选作萃取剂,因为它具有低水溶性,高稳定性,并且比胺型萃取剂更环保。对于反应性萃取过程中使用的其他胺萃取剂,文献中缺乏关于用DOA提取这些酸的研究。将萃取剂溶解在具有不同化学结构的九种不同溶剂(丁醇,癸醇,辛醇,异戊醇,辛烷,癸烷,甲基异丁基酮和二异丁基酮)中。所有实验均在298.15 K下进行。使用分布系数(D),负载率(Z)和提取率(%E)。观察到,与之相比,使用DOA可以实现相当数量的柠檬酸和马来酸提取(根据用于柠檬酸和马来酸的稀释剂,分别在13.95–99.19%和31.94–99.41%的范围内)。用TOPO达到的效果(根据柠檬酸和马来酸的稀释剂分别在0.81–67.12%和16.25–89.33%的范围内)。
更新日期:2017-12-27
中文翻译:

使用磷键合萃取剂三正辛基膦氧化物和仲胺二辛胺从水溶液中萃取柠檬酸和马来酸
这项研究的目的是研究使用不同的溶剂萃取剂混合物从其水溶液中萃取柠檬酸和马来酸,以便找到最有效的萃取成分。选择柠檬酸和马来酸作为羧酸,因为它们在工业过程中具有商业价值。通过反应萃取,一种有前途的液-液萃取技术,使用三辛基氧化膦(TOPO)和称为二辛胺(DOA)的仲胺,可以从水溶液中回收羧酸。TOPO已被选作萃取剂,因为它具有低水溶性,高稳定性,并且比胺型萃取剂更环保。对于反应性萃取过程中使用的其他胺萃取剂,文献中缺乏关于用DOA提取这些酸的研究。将萃取剂溶解在具有不同化学结构的九种不同溶剂(丁醇,癸醇,辛醇,异戊醇,辛烷,癸烷,甲基异丁基酮和二异丁基酮)中。所有实验均在298.15 K下进行。使用分布系数(D),负载率(Z)和提取率(%E)。观察到,与之相比,使用DOA可以实现相当数量的柠檬酸和马来酸提取(根据用于柠檬酸和马来酸的稀释剂,分别在13.95–99.19%和31.94–99.41%的范围内)。用TOPO达到的效果(根据柠檬酸和马来酸的稀释剂分别在0.81–67.12%和16.25–89.33%的范围内)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号