当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
cGMP Binding Domain D Mediates a Unique Activation Mechanism in Plasmodium falciparum PKG
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1021/acsinfecdis.7b00222 Eugen Franz 1 , Matthias J. Knape 1 , Friedrich W. Herberg 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1021/acsinfecdis.7b00222 Eugen Franz 1 , Matthias J. Knape 1 , Friedrich W. Herberg 1
Affiliation
|
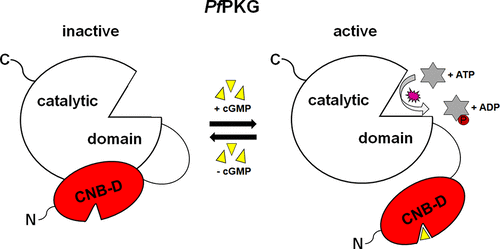
cGMP-dependent protein kinase from Plasmodium falciparum (PfPKG) plays a crucial role in the sexual as well as the asexual proliferation of this human malaria causing parasite. However, function and regulation of PfPKG are largely unknown. Previous studies showed that the domain organization of PfPKG significantly differs from human PKG (hPKG) and indicated a critical role of the cyclic nucleotide binding domain D (CNB-D). We identified a novel mechanism, where the CNB-D controls activation and regulation of the parasite specific protein kinase. Here, kinase activity is not dependent on a pseudosubstrate autoinhibitory sequence (IS), as reported for human PKG. A construct lacking the putative IS and containing only the CNB-D and the catalytic domain is inactive in the absence of cGMP and can efficiently be activated with cGMP. On the basis of structural evidence, we describe a regulatory mechanism, whereby cGMP binding to CNB-D induces a conformational change involving the αC-helix of the CNB-D. The inactive state is defined by a unique interaction between Asp597 of the catalytic domain and Arg528 of the αC-helix. The same arginine (R528), however, stabilizes cGMP binding by interacting with Tyr480 of the phosphate binding cassette (PBC). This represents the active state of PfPKG. Our results unveil fundamental differences in the activation mechanism between PfPKG and hPKG, building the basis for the development of strategies for targeted drug design in fighting malaria.
中文翻译:

cGMP结合域D在恶性疟原虫PKG中介导独特的激活机制
恶性疟原虫(Pf PKG)的cGMP依赖性蛋白激酶在这种引起疟原虫的人类疟疾的性繁殖和无性繁殖中起着至关重要的作用。但是,Pf PKG的功能和调节在很大程度上尚不清楚。先前的研究表明,Pf PKG的结构域域与人类PKG显着不同(hPKG),并指出了环状核苷酸结合结构域D(CNB-D)的关键作用。我们确定了一种新的机制,其中CNB-D控制寄生虫特异性蛋白激酶的激活和调节。如人类PKG所报道的,此处的激酶活性不依赖于假底物自抑制序列(IS)。缺少推定的IS且仅包含CNB-D和催化结构域的构建体在不存在cGMP的情况下是无活性的,并且可以被cGMP有效激活。根据结构证据,我们描述了一种调节机制,其中cGMP与CNB-D的结合诱导了涉及CNB-D的αC-螺旋的构象变化。失活状态是由催化结构域的Asp597和αC-螺旋的Arg528之间的唯一相互作用定义的。同样的精氨酸(R528),通过与磷酸结合盒(PBC)的Tyr480相互作用来稳定cGMP结合。这代表了活动状态Pf PKG。我们的研究结果揭示了Pf PKG和h PKG在激活机制上的根本差异,为开发抗击疟疾的靶向药物设计策略奠定了基础。
更新日期:2017-12-18
中文翻译:

cGMP结合域D在恶性疟原虫PKG中介导独特的激活机制
恶性疟原虫(Pf PKG)的cGMP依赖性蛋白激酶在这种引起疟原虫的人类疟疾的性繁殖和无性繁殖中起着至关重要的作用。但是,Pf PKG的功能和调节在很大程度上尚不清楚。先前的研究表明,Pf PKG的结构域域与人类PKG显着不同(hPKG),并指出了环状核苷酸结合结构域D(CNB-D)的关键作用。我们确定了一种新的机制,其中CNB-D控制寄生虫特异性蛋白激酶的激活和调节。如人类PKG所报道的,此处的激酶活性不依赖于假底物自抑制序列(IS)。缺少推定的IS且仅包含CNB-D和催化结构域的构建体在不存在cGMP的情况下是无活性的,并且可以被cGMP有效激活。根据结构证据,我们描述了一种调节机制,其中cGMP与CNB-D的结合诱导了涉及CNB-D的αC-螺旋的构象变化。失活状态是由催化结构域的Asp597和αC-螺旋的Arg528之间的唯一相互作用定义的。同样的精氨酸(R528),通过与磷酸结合盒(PBC)的Tyr480相互作用来稳定cGMP结合。这代表了活动状态Pf PKG。我们的研究结果揭示了Pf PKG和h PKG在激活机制上的根本差异,为开发抗击疟疾的靶向药物设计策略奠定了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号