Dyes and Pigments ( IF 4.1 ) Pub Date : 2017-12-27 , DOI: 10.1016/j.dyepig.2017.12.042 Ruomeng Duan , Manfred Wagner , Klaus Müllen , Chen Li
|
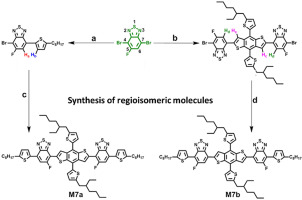
Two constitutional π-conjugated small molecular isomers M7a and M7b were prepared by regioselective Stille reactions. M7a and M7b have the same D1-A-D2-A-D1 structure configuration, where D1, A, and D2 are hexylthiophene, fluorobenzothiadiazole (FBT), and benzodithiophene (BDT), correspondingly. Nevertheless, they differ in the mode of fluoro substitution. Namely, F-substituents are located proximal and distal to the BDT central unit for M7a and M7b, respectively. M7a and M7b showed significant dissimilarities in their thermal, optical, electrochemical characteristics. Particularly, with the same device construction and under the identical test conditions, the organic solar cells based on M7a and M7b exhibited different photovoltaic performance. This study investigated the relationship between the substitution mode of fluorine atoms and the molecular properties, which will provide guidelines for future structural design regarding fine tuning of molecular properties and selection of regioisomeric π-conjugated small molecules.
中文翻译:

D1-A-D2-A-D1型带有苯并二噻吩,苯并噻二唑和噻吩的结构性π共轭小分子异构体
通过区域选择性Stille反应制备了两个组成的π共轭的小分子异构体M7a和M7b。M7a和M7b具有相同的D1-A-D2-A-D1结构配置,其中D1,A和D2分别是己基噻吩,氟苯并噻二唑(FBT)和苯并二噻吩(BDT)。然而,它们在氟取代的方式上有所不同。即,对于M7a和M7b,F取代基分别位于BDT中央单元的近端和远端。M7a和M7b在热,光学,电化学特性上显示出极大的不同。特别地,在相同的器件构造和相同的测试条件下,基于M7a和M7b的有机太阳能电池表现出不同的光伏性能。这项研究调查了氟原子的取代模式与分子性质之间的关系,这将为将来的结构设计提供指导,以进行分子性质的微调和区域异构π共轭小分子的选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号