当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
5-heptyl-1,3,4-oxadiazole-2-thione: Synthesis and flotation mechanism to chalcopyrite
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2018-05-01 , DOI: 10.1016/j.jiec.2017.12.031 Yaoguo Huang , Guangyi Liu , Longqun Ma , Jun Liu
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2018-05-01 , DOI: 10.1016/j.jiec.2017.12.031 Yaoguo Huang , Guangyi Liu , Longqun Ma , Jun Liu
|
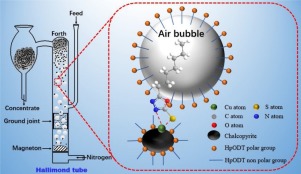
Abstract In this article, 5-heptyl-1,3,4-oxadiazole-2-thione (HpODT) surfactant was synthesized and originally recommended as a collector for flotation recovery of chalcopyrite, bornite and pyrite. The micro-flotation findings indicated that compared with traditional collectors such as sodium isobutyl xanthate (SIBX) and sodium hexyl xanthate (SHX), HpODT possessed superior flotation affinity to chalcopyrite and bornite, and selectivity against pyrite under alkaline circumstances. After HpODT treatment, the contact angle of chalcopyrite increased, suggesting an improved hydrophobicity of chalcopyrite surfaces. Adsorption thermodynamic and kinetic analyses suggested that chalcopyrite adsorption of HpODT was a spontaneous-endothermic chemisorption process, and the ΔH (change of enthalpy), ΔS (change of entropy), ΔG (change of free energy) and Ea (activation energy) were 21.06 kJ mol−1, 173.63 J mol−1 K−1, −30.77 kJ mol−1 (298 K) and 20.19 kJ mol−1, respectively. The results of zeta potential hinted that HpODT chemisorbed on chalcopyrite surfaces. UV–vis spectra clearly observed that HpODT selectively reacted with Cu+ or Cu2+, not with Fe2+ or Fe3+ ions. FTIR spectra inferred that HpODT–Cu surface complexes were formed on chalcopyrite by reaction of HpODT’s C N NH C( S) O group with surface copper atoms to build Cu S and Cu N bonds.
中文翻译:

5-heptyl-1,3,4-oxadiazole-2-thione:黄铜矿的合成及浮选机理
摘要 本文合成了5-庚基-1,3,4-恶二唑-2-硫酮(HpODT)表面活性剂,最初推荐作为黄铜矿、斑铜矿和黄铁矿浮选回收的捕收剂。微浮选结果表明,与异丁基黄原酸钠(SIBX)和己基黄原酸钠(SHX)等传统捕收剂相比,HpODT对黄铜矿和斑铜矿具有优异的浮选亲和力,在碱性环境下对黄铁矿具有选择性。HpODT 处理后,黄铜矿的接触角增加,表明黄铜矿表面的疏水性提高。吸附热力学和动力学分析表明,HpODT 对黄铜矿的吸附是一个自发吸热的化学吸附过程,ΔH(焓变)、ΔS(熵变)、ΔG(自由能变化)和 Ea(活化能)分别为 21.06 kJ mol-1、173.63 J mol-1 K-1、-30.77 kJ mol-1 (298 K) 和 20.19 kJ mol-1。Zeta 电位的结果表明 HpODT 化学吸附在黄铜矿表面。UV-vis 光谱清楚地观察到 HpODT 选择性地与 Cu+ 或 Cu2+ 反应,而不是与 Fe2+ 或 Fe3+ 离子反应。FTIR 光谱推断 HpODT-Cu 表面复合物是通过 HpODT 的 CN NH C( S) O 基团与表面铜原子反应形成 Cu S 和 Cu N 键而在黄铜矿上形成的。
更新日期:2018-05-01
中文翻译:

5-heptyl-1,3,4-oxadiazole-2-thione:黄铜矿的合成及浮选机理
摘要 本文合成了5-庚基-1,3,4-恶二唑-2-硫酮(HpODT)表面活性剂,最初推荐作为黄铜矿、斑铜矿和黄铁矿浮选回收的捕收剂。微浮选结果表明,与异丁基黄原酸钠(SIBX)和己基黄原酸钠(SHX)等传统捕收剂相比,HpODT对黄铜矿和斑铜矿具有优异的浮选亲和力,在碱性环境下对黄铁矿具有选择性。HpODT 处理后,黄铜矿的接触角增加,表明黄铜矿表面的疏水性提高。吸附热力学和动力学分析表明,HpODT 对黄铜矿的吸附是一个自发吸热的化学吸附过程,ΔH(焓变)、ΔS(熵变)、ΔG(自由能变化)和 Ea(活化能)分别为 21.06 kJ mol-1、173.63 J mol-1 K-1、-30.77 kJ mol-1 (298 K) 和 20.19 kJ mol-1。Zeta 电位的结果表明 HpODT 化学吸附在黄铜矿表面。UV-vis 光谱清楚地观察到 HpODT 选择性地与 Cu+ 或 Cu2+ 反应,而不是与 Fe2+ 或 Fe3+ 离子反应。FTIR 光谱推断 HpODT-Cu 表面复合物是通过 HpODT 的 CN NH C( S) O 基团与表面铜原子反应形成 Cu S 和 Cu N 键而在黄铜矿上形成的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号