Journal of Power Sources ( IF 8.1 ) Pub Date : 2017-12-22 , DOI: 10.1016/j.jpowsour.2017.11.092 Naoya Kurimoto , Ryo Omoda , Tomonobu Mizumo , Seitaro Ito , Yuichi Aihara , Takahito Itoh
|
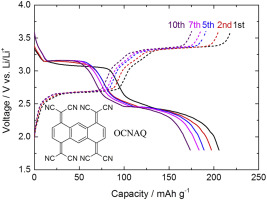
Quinoid compounds are important candidates of organic active materials for lithium-ion batteries. However, its high solubility to organic electrolyte solutions and low redox potential are known as their major drawbacks. To circumvent these issues, we have designed and synthesized a tandem-tetracyanoquinonedimethane type cathode-active material, 11,11,12,12,13,13,14,14-octacyano-1,4,5,8-anthradiquinotetramethane (OCNAQ), that has four redox sites per molecule, high redox potential and suppressed solubility to electrolyte solution. Synthesized OCNAQ has been found to have two-step redox reactions by cyclic voltammetry, and each step consists of two-electron reactions. During charge-discharge tests using selected organic cathode-active materials with a lithium metal anode, the cell voltages obtained from OCNAQ are higher than those for 11,11-dicyanoanthraquinone methide (AQM) as expected, due to the strong electron-withdrawing effect of the cyano groups. Unfortunately, even with the use of the organic active material, the issue of dissolution to the electrolyte solution cannot be suppressed completely; however, appropriate choice of the electrolyte solutions, glyme-based electrolyte solutions in this study, give considerable improvement of the cycle retention (98% and 56% at 10 and 100 cycles at 0.5C, respectively). The specific capacity and energy density obtained in this study are 206 mAh g−1 and 554 mWh g−1 with respect to the cathode active material.
中文翻译:

锂二次电池正极活性物质的四电子转移串联四氰基喹二甲烷
醌类化合物是锂离子电池有机活性材料的重要候选物。然而,其对有机电解质溶液的高溶解度和低氧化还原电势是其主要缺点。为了解决这些问题,我们设计并合成了串联的四氰基醌二甲烷型正极活性材料11,11,12,12,13,13,14,14-十八烷基-1,4,5,8-蒽醌四甲烷(OCNAQ) ,每个分子具有四个氧化还原位点,氧化还原电位高,并且对电解质溶液的溶解度较低。通过循环伏安法已经发现合成的OCNAQ具有两步氧化还原反应,并且每一步都由两电子反应组成。在使用选定的有机阴极活性材料和锂金属阳极进行充放电测试期间,从OCNAQ获得的电池电压高于11的电池电压 如预期的那样,由于氰基具有很强的吸电子作用,因此11-二氰基蒽醌甲基化物(AQM)。不幸的是,即使使用有机活性材料,也不能完全抑制在电解质溶液中的溶解问题。然而,电解质溶液的合适的选择,在该研究中基于甘醇二甲醚 - 电解质溶液,得到(在分别以0.5C 10个100次循环,98%和56%)相当大的改进循环保持的。在这项研究中获得的比容量和能量密度为206 mAh g 电解质溶液的合适的选择,在该研究中基于甘醇二甲醚 - 电解质溶液,得到(在分别以0.5C 10个100次循环,98%和56%)相当大的改进循环保持的。在这项研究中获得的比容量和能量密度为206 mAh g 电解质溶液的合适的选择,在该研究中基于甘醇二甲醚 - 电解质溶液,得到(在分别以0.5C 10个100次循环,98%和56%)相当大的改进循环保持的。在这项研究中获得的比容量和能量密度为206 mAh g相对于正极活性物质为-1和554mWh g -1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号