当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibitors of HIV-1 Attachment: The Discovery and Development of Temsavir and its Prodrug Fostemsavir
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01337 Nicholas A. Meanwell , Mark R. Krystal , Beata Nowicka-Sans , David R. Langley , David A. Conlon 1 , Martin D. Eastgate 1 , Dennis M. Grasela 2 , Peter Timmins 3 , Tao Wang , John F. Kadow
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01337 Nicholas A. Meanwell , Mark R. Krystal , Beata Nowicka-Sans , David R. Langley , David A. Conlon 1 , Martin D. Eastgate 1 , Dennis M. Grasela 2 , Peter Timmins 3 , Tao Wang , John F. Kadow
Affiliation
|
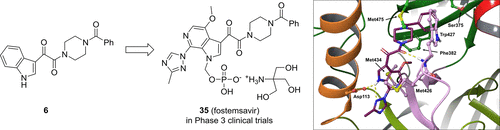
Human immunodeficiency virus-1 (HIV-1) infection currently requires lifelong therapy with drugs that are used in combination to control viremia. The indole-3-glyoxamide 6 was discovered as an inhibitor of HIV-1 infectivity using a phenotypic screen and derivatives of this compound were found to interfere with the HIV-1 entry process by stabilizing a conformation of the virus gp120 protein not recognized by the host cell CD4 receptor. An extensive optimization program led to the identification of temsavir (31), which exhibited an improved antiviral and pharmacokinetic profile compared to 6 and was explored in phase 3 clinical trials as the phosphonooxymethyl derivative fostemsavir (35), a prodrug designed to address dissolution- and solubility-limited absorption issues. In this drug annotation, we summarize the structure–activity and structure–liability studies leading to the discovery of 31 and the clinical studies conducted with 35 that entailed the development of an extended release formulation suitable for phase 3 clinical trials.
中文翻译:

HIV-1附着抑制剂:坦沙韦及其前药福斯特沙韦的发现与发展
目前,人类免疫缺陷病毒1(HIV-1)感染需要终身治疗,并需要联合使用药物来控制病毒血症。使用表型筛选发现了吲哚-3-乙二酰胺6作为HIV-1感染性的抑制剂,并且发现该化合物的衍生物通过稳定病毒gp120蛋白无法识别的构象来干扰HIV-1进入过程。宿主细胞CD4受体。广泛的优化程序导致了temsavir的鉴定(31),与6相比,temsavir的抗病毒和药代动力学特性有所改善,并在3期临床试验中被探索为膦酰氧甲基衍生物fostemsavir(35),一种旨在解决溶解度和溶解度受限的吸收问题的前药。在该药物注释中,我们总结了导致发现31的结构-活性和结构-可靠性研究,以及对35进行的临床研究,这些研究涉及开发适用于3期临床试验的缓释制剂。
更新日期:2017-12-22
中文翻译:

HIV-1附着抑制剂:坦沙韦及其前药福斯特沙韦的发现与发展
目前,人类免疫缺陷病毒1(HIV-1)感染需要终身治疗,并需要联合使用药物来控制病毒血症。使用表型筛选发现了吲哚-3-乙二酰胺6作为HIV-1感染性的抑制剂,并且发现该化合物的衍生物通过稳定病毒gp120蛋白无法识别的构象来干扰HIV-1进入过程。宿主细胞CD4受体。广泛的优化程序导致了temsavir的鉴定(31),与6相比,temsavir的抗病毒和药代动力学特性有所改善,并在3期临床试验中被探索为膦酰氧甲基衍生物fostemsavir(35),一种旨在解决溶解度和溶解度受限的吸收问题的前药。在该药物注释中,我们总结了导致发现31的结构-活性和结构-可靠性研究,以及对35进行的临床研究,这些研究涉及开发适用于3期临床试验的缓释制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号