当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Silico Fragment-Based Design Identifies Subfamily B1 Metallo-β-lactamase Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01728 Ricky Cain 1 , Jürgen Brem 2 , David Zollman 2 , Michael A. McDonough 2 , Rachel M. Johnson 1 , James Spencer 3 , Anne Makena 2 , Martine I. Abboud 2 , Samuel Cahill 2 , Sook Y. Lee 2, 4 , Peter J. McHugh 4 , Christopher J. Schofield 2 , Colin W. G. Fishwick 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01728 Ricky Cain 1 , Jürgen Brem 2 , David Zollman 2 , Michael A. McDonough 2 , Rachel M. Johnson 1 , James Spencer 3 , Anne Makena 2 , Martine I. Abboud 2 , Samuel Cahill 2 , Sook Y. Lee 2, 4 , Peter J. McHugh 4 , Christopher J. Schofield 2 , Colin W. G. Fishwick 1
Affiliation
|
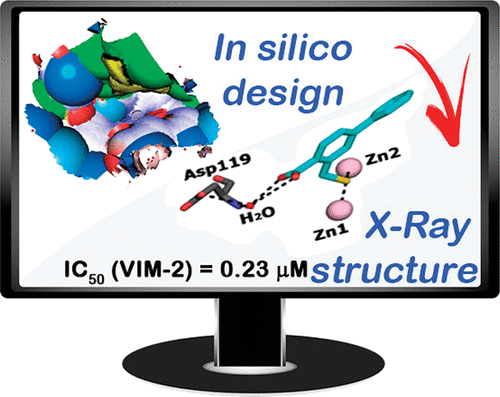
Zinc ion-dependent β-lactamases (MBLs) catalyze the hydrolysis of almost all β-lactam antibiotics and resist the action of clinically available β-lactamase inhibitors. We report how application of in silico fragment-based molecular design employing thiol-mediated metal anchorage leads to potent MBL inhibitors. The new inhibitors manifest potent inhibition of clinically important B1 subfamily MBLs, including the widespread NDM-1, IMP-1, and VIM-2 enzymes; with lower potency, some of them also inhibit clinically relevant Class A and D serine-β-lactamases. The inhibitors show selectivity for bacterial MBL enzymes compared to that for human MBL fold nucleases. Cocrystallization of one inhibitor, which shows potentiation of Meropenem activity against MBL-expressing Enterobacteriaceae, with VIM-2 reveals an unexpected binding mode, involving interactions with residues from conserved active site bordering loops.
中文翻译:

基于计算机片段的设计鉴定了亚家族B1金属β-内酰胺酶抑制剂
锌离子依赖性β-内酰胺酶(MBL)催化几乎所有β-内酰胺抗生素的水解,并抵抗临床可用的β-内酰胺酶抑制剂的作用。我们报告了如何应用基于硫醇介导的金属锚固的基于计算机模拟片段的分子设计导致有效的MBL抑制剂。新的抑制剂表现出对临床上重要的B1亚家族MBL的有效抑制作用,包括广泛的NDM-1,IMP-1和VIM-2酶。具有较低的效力,它们中的一些还抑制临床上相关的A和D类丝氨酸-β-内酰胺酶。与人MBL折叠核酸酶相比,该抑制剂对细菌MBL酶具有选择性。一种抑制剂的共结晶,该抑制剂显示美洛培南对表达MBL的肠杆菌科细菌具有增强活性, 使用VIM-2时,发现了意外的结合模式,涉及与保守的活性位点边界环的残基相互作用。
更新日期:2018-01-10
中文翻译:

基于计算机片段的设计鉴定了亚家族B1金属β-内酰胺酶抑制剂
锌离子依赖性β-内酰胺酶(MBL)催化几乎所有β-内酰胺抗生素的水解,并抵抗临床可用的β-内酰胺酶抑制剂的作用。我们报告了如何应用基于硫醇介导的金属锚固的基于计算机模拟片段的分子设计导致有效的MBL抑制剂。新的抑制剂表现出对临床上重要的B1亚家族MBL的有效抑制作用,包括广泛的NDM-1,IMP-1和VIM-2酶。具有较低的效力,它们中的一些还抑制临床上相关的A和D类丝氨酸-β-内酰胺酶。与人MBL折叠核酸酶相比,该抑制剂对细菌MBL酶具有选择性。一种抑制剂的共结晶,该抑制剂显示美洛培南对表达MBL的肠杆菌科细菌具有增强活性, 使用VIM-2时,发现了意外的结合模式,涉及与保守的活性位点边界环的残基相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号