Talanta ( IF 5.6 ) Pub Date : 2017-12-22 , DOI: 10.1016/j.talanta.2017.12.062 Qianqian Wu , Kangnan Wang , Zian Wang , Yatong Sun , Duxia Cao , Zhiqiang Liu , Ruifang Guan , Songfang Zhao , Xueying Yu
|
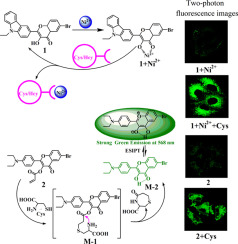
Two 3-hydroxyflavone derivatives as one- and two-photon fluorescent chemosensors for cysteine (Cys) and homocysteine (Hcy) were synthesized. The recognition properties and mechanism of the chemosensors for Cys and Hcy were investigated systematically. The experiment results indicate that 3-hydroxyflavone compound 1 (6-bromo-2-(9-ethyl-9H-carbazol-3-yl)-3-hydroxy-chromen-4-one) after the addition of nickel ions exhibits good recognition properties for Cys and Hcy with fluorescence enhancement and 65 nm absorption peak blue shift based on nickel displacement reaction mechanism. The detection limits (DL) with fluorescence as detected signal are 4.06 × 10−3 µM (Cys, linear range of 10–80 µM) and 5.8 × 10−3 µM (Hcy, linear range of 10–100 µM), respectively. But acrylate substituted 3-hydroxyflavone compound 2 (4-oxo-2-(4-diethylamino-phenyl)-4H-chromen-3-yl acrylate) can specially identify Cys with fluorescence turn-on (DL = 1.87 × 10−3 µM, linear range of 4–22 µM) based on Cys leading to acrylate hydrolysis mechanism and succedent excited-state intramolecular proton transfer process of 3-hydroxyflavone compound. Then Cys and Hcy biological thiols can be recognized at one time by these two 3-hydroxyflavone derivatives. The bioimaging experiment indicates that both the compounds can be successfully applied to the detection of Cys/Hcy in living cells and compound 2 also can be applied to bioimaging Cys in zebrafish by one- and two-photon fluorescence mode. Then these two compounds have a potential in the application of biological sample analysis.
中文翻译:

两种3-羟基黄酮衍生物作为活细胞中半胱氨酸和高半胱氨酸的双光子荧光开启化学传感器
合成了两个3-羟基黄酮衍生物,作为半胱氨酸(Cys)和高半胱氨酸(Hcy)的单光子和双光子荧光化学传感器。系统地研究了化学传感器对Cys和Hcy的识别特性和机理。实验结果表明,添加镍离子后的3-羟基黄酮类化合物1(6-溴-2-(9-乙基-9 H-咔唑-3-基)-3-羟基-铬烯-4-酮)表现出良好的性能。镍置换反应机理的荧光增强和65 nm吸收峰蓝移识别Cys和Hcy的特性。以荧光作为检测信号的检测限(DL)为4.06×10 -3 µM(Cys,线性范围为10–80 µM)和5.8×10 -3 µM(Hcy,线性范围为10–100 µM)。但是丙烯酸酯取代的3-羟基黄酮化合物2(丙烯酸4-氧代-2-(4-二乙基氨基-苯基)-4 H-铬-3-基丙烯酸酯)可以特别识别具有荧光开启性的Cys(DL = 1.87×10 -3 µM,线性范围为4-22 µM)基于Cys,导致丙烯酸酯水解机理和3-羟基黄酮化合物的成功激发态分子内质子转移过程。然后,这两种3-羟基黄酮衍生物可以同时识别Cys和Hcy生物硫醇。生物成像实验表明,这两种化合物均可成功用于活细胞和化合物2中Cys / Hcy的检测。也可以通过单光子和双光子荧光模式应用于斑马鱼的生物成像半胱氨酸。那么这两种化合物在生物样品分析中的应用就有潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号