当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Histidine–Lysine Axial Ligand Switching in a Hemoglobin: A Role for Heme Propionates
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.biochem.7b01155 Dillon B. Nye 1 , Matthew R. Preimesberger 1 , Ananya Majumdar 2 , Juliette T. J. Lecomte 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.biochem.7b01155 Dillon B. Nye 1 , Matthew R. Preimesberger 1 , Ananya Majumdar 2 , Juliette T. J. Lecomte 1
Affiliation
|
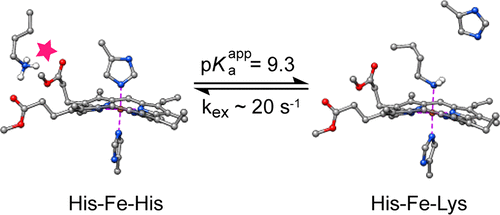
The hemoglobin of Synechococcus sp. PCC 7002, GlbN, is a monomeric group I truncated protein (TrHb1) that coordinates the heme iron with two histidine ligands at neutral pH. One of these is the distal histidine (His46), a residue that can be displaced by dioxygen and other small molecules. Here, we show with mutagenesis, electronic absorption spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy that at high pH and exclusively in the ferrous state, Lys42 competes with His46 for the iron coordination site. When b heme is originally present, the population of the lysine-bound species remains too small for detailed characterization; however, the population can be increased significantly by using dimethyl-esterified heme. Electronic absorption and NMR spectroscopies showed that the reversible ligand switching process occurs with an apparent pKa of 9.3 and a Lys-ligated population of ∼60% at the basic pH limit in the modified holoprotein. The switching rate, which is slow on the chemical shift time scale, was estimated to be 20–30 s–1 by NMR exchange spectroscopy. Lys42–His46 competition and attendant conformational rearrangement appeared to be related to weakened bis-histidine ligation and enhanced backbone dynamics in the ferrous protein. The pH- and redox-dependent ligand exchange process observed in GlbN illustrates the structural plasticity allowed by the TrHb1 fold and demonstrates the importance of electrostatic interactions at the heme periphery for achieving axial ligand selection. An analogy is drawn to the alkaline transition of cytochrome c, in which Lys–Met competition is detected at alkaline pH, but, in contrast to GlbN, in the ferric state only.
中文翻译:

组氨酸-赖氨酸轴向配体在血红蛋白中的转换:血红素丙酸酯的作用
Synechococcus sp。的血红蛋白。PCC 7002,GlbN,是I类单体的截短蛋白(TrHb1),可在中性pH下将血红素铁与两个组氨酸配体配位。其中之一是远端组氨酸(His46),该残基可被双氧和其他小分子置换。在这里,我们通过诱变,电子吸收光谱和核磁共振(NMR)光谱显示,在高pH且仅在亚铁状态下,Lys42与His46竞争铁配位位点。当b血红素最初存在,与赖氨酸结合的物种的数量仍然太少,无法进行详细表征。但是,使用二甲基酯化血红素可以显着增加种群。电子吸收和NMR光谱表明,在修饰的全蛋白中,在碱性pH值下,可逆配体转换过程发生,表观p K a为9.3,Lys连接的人群为〜60%。开关速率在化学位移时间范围内较慢,估计为20–30 s –1通过NMR交换光谱法。Lys42–His46竞争和伴随的构象重排似乎与弱化的双组氨酸连接和铁蛋白中骨架动力学的增强有关。在GlbN中观察到的pH和氧化还原依赖性配体交换过程说明了TrHb1折叠所允许的结构可塑性,并证明了血红素外围的静电相互作用对于实现轴向配体选择的重要性。类比于细胞色素c的碱性转变,其中在碱性pH下检测到Lys–Met竞争,但与GlbN相反,仅在铁态下。
更新日期:2018-01-10
中文翻译:

组氨酸-赖氨酸轴向配体在血红蛋白中的转换:血红素丙酸酯的作用
Synechococcus sp。的血红蛋白。PCC 7002,GlbN,是I类单体的截短蛋白(TrHb1),可在中性pH下将血红素铁与两个组氨酸配体配位。其中之一是远端组氨酸(His46),该残基可被双氧和其他小分子置换。在这里,我们通过诱变,电子吸收光谱和核磁共振(NMR)光谱显示,在高pH且仅在亚铁状态下,Lys42与His46竞争铁配位位点。当b血红素最初存在,与赖氨酸结合的物种的数量仍然太少,无法进行详细表征。但是,使用二甲基酯化血红素可以显着增加种群。电子吸收和NMR光谱表明,在修饰的全蛋白中,在碱性pH值下,可逆配体转换过程发生,表观p K a为9.3,Lys连接的人群为〜60%。开关速率在化学位移时间范围内较慢,估计为20–30 s –1通过NMR交换光谱法。Lys42–His46竞争和伴随的构象重排似乎与弱化的双组氨酸连接和铁蛋白中骨架动力学的增强有关。在GlbN中观察到的pH和氧化还原依赖性配体交换过程说明了TrHb1折叠所允许的结构可塑性,并证明了血红素外围的静电相互作用对于实现轴向配体选择的重要性。类比于细胞色素c的碱性转变,其中在碱性pH下检测到Lys–Met竞争,但与GlbN相反,仅在铁态下。











































 京公网安备 11010802027423号
京公网安备 11010802027423号