当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glutamate Induced Thermal Equilibrium Intermediate and Counteracting Effect on Chemical Denaturation of Proteins
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acs.jpcb.7b10561 Bramhini Anumalla 1 , N. Prakash Prabhu 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acs.jpcb.7b10561 Bramhini Anumalla 1 , N. Prakash Prabhu 1
Affiliation
|
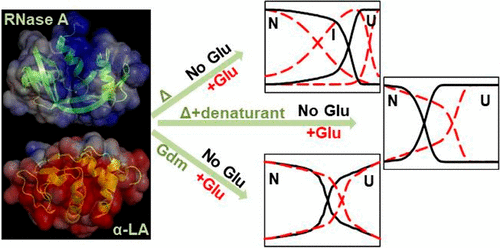
When organisms are subjected to stress conditions, one of their adaptive responses is accumulation of small organic molecules called osmolytes. These osmolytes affect the structure and stability of the biological macromolecules including proteins. The present study examines the effect of a negatively charged amino acid osmolyte, glutamate (Glu), on two model proteins, ribonuclease A (RNase A) and α-lactalbumin (α-LA), which have positive and negative surface charges at pH 7, respectively. These proteins follow two-state unfolding transitions during both heat and chemical induced denaturation processes. The addition of Glu stabilizes the proteins against temperature and induces an early equilibrium intermediate during unfolding. The stability is found to be enthalpy-driven, and the free energy of stabilization is more for α-LA compared to RNase A. The decrease in the partial molar volume and compressibility of both of the proteins in the presence of Glu suggests that the proteins attain a more compact state through surface hydration which could provide a more stable conformation. This is also supported by molecule dynamic simulation studies which demonstrate that the water density around the proteins is increased upon the addition of Glu. Further, the intermediates could be completely destabilized by lower concentrations (∼0.5 M) of guanidinium chloride and salt. However, urea subverts the Glu-induced intermediate formed by α-LA, whereas it only slightly destabilizes in the case of RNase A which has a positive surface charge and could possess charge–charge interactions with Glu. This suggests that, apart from hydration, columbic interactions might also contribute to the stability of the intermediate. Gdm-induced denaturation of RNase A and α-LA in the absence and the presence of Glu at different temperatures was carried out. These results also show the Glu-induced stabilization of both of the proteins; however, all of the unfolding transitions followed two-state transitions during chemical denaturation. The extent of stability exerted by Glu is higher for RNase A at higher temperature, whereas it provides more stability for α-LA at lower temperature. Thus, the experiments indicate that Glu induces a thermal equilibrium intermediate and increases the thermodynamic stability of proteins irrespective of their surface charges. The extent of stability varies between the proteins in a temperature-dependent manner.
中文翻译:

谷氨酸诱导的热平衡中间体及其对蛋白质化学变性的抵消作用
当生物体遭受压力条件时,它们的适应性反应之一就是称为渗透液的有机小分子的积累。这些渗透物影响包括蛋白质在内的生物大分子的结构和稳定性。本研究检查了带负电荷的氨基酸渗透物谷氨酸(Glu)对两种模型蛋白核糖核酸酶A(RNase A)和α-乳清蛋白(α-LA)的影响,它们在pH 7时具有正负表面电荷, 分别。这些蛋白质在热和化学诱导的变性过程中均遵循两个状态的展开转变。Glu的添加可稳定蛋白质的温度,并在展开过程中诱导早期的平衡中间产物。发现稳定性是由焓驱动的,与RNase A相比,α-LA的稳定自由能更大。在存在Glu的情况下,两种蛋白质的部分摩尔体积和可压缩性的降低表明,这些蛋白质通过表面水合达到了更紧密的状态,这可以提供更稳定的构象。分子动力学模拟研究也证明了这一点,该研究表明,添加Glu后,蛋白质周围的水密度会增加。此外,中间体可能会因较低浓度(约0.5 M)的氯化胍和盐而完全不稳定。但是,尿素会破坏由α-LA形成的Glu诱导的中间产物,而在RNase A的情况下,尿素只会稍微失稳,而RNase A具有正的表面电荷,并可能与Glu发生电荷-电荷相互作用。这表明,除了水合作用外,哥伦比亚的相互作用也可能有助于中间体的稳定性。在不存在和存在Glu的情况下,在不同温度下进行Gdm诱导的RNase A和α-LA的变性。这些结果还显示了Glu诱导的两种蛋白质的稳定。然而,在化学变性过程中,所有展开的转变都遵循两个状态的转变。对于较高温度的RNase A,Glu发挥的稳定性程度较高,而对于较低温度的α-LA,它提供的稳定性更高。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。在不存在和存在Glu的情况下,在不同温度下进行Gdm诱导的RNase A和α-LA的变性。这些结果还显示了Glu诱导的两种蛋白质的稳定。然而,在化学变性过程中,所有展开的转变都遵循两个状态的转变。对于较高温度的RNase A,Glu发挥的稳定性程度较高,而对于较低温度的α-LA,它提供的稳定性更高。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。在不存在和存在Glu的情况下,在不同温度下进行Gdm诱导的RNase A和α-LA的变性。这些结果还显示了Glu诱导的两种蛋白质的稳定。然而,在化学变性过程中,所有展开的转变都遵循两个状态的转变。对于较高温度的RNase A,Glu发挥的稳定性程度较高,而对于较低温度的α-LA,它提供的稳定性更高。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。这些结果还显示了Glu诱导的两种蛋白质的稳定。然而,在化学变性过程中,所有展开的转变都遵循两个状态的转变。对于较高温度的RNase A,Glu发挥的稳定性程度较高,而对于较低温度的α-LA,它提供的稳定性更高。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。这些结果还显示了Glu诱导的两种蛋白质的稳定。然而,在化学变性过程中,所有展开的转变都遵循两个状态的转变。对于较高温度的RNase A,Glu发挥的稳定性程度较高,而对于较低温度的α-LA,它提供的稳定性更高。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。相反,它在较低温度下为α-LA提供了更高的稳定性。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。相反,它在较低温度下为α-LA提供了更高的稳定性。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。
更新日期:2018-01-09
中文翻译:

谷氨酸诱导的热平衡中间体及其对蛋白质化学变性的抵消作用
当生物体遭受压力条件时,它们的适应性反应之一就是称为渗透液的有机小分子的积累。这些渗透物影响包括蛋白质在内的生物大分子的结构和稳定性。本研究检查了带负电荷的氨基酸渗透物谷氨酸(Glu)对两种模型蛋白核糖核酸酶A(RNase A)和α-乳清蛋白(α-LA)的影响,它们在pH 7时具有正负表面电荷, 分别。这些蛋白质在热和化学诱导的变性过程中均遵循两个状态的展开转变。Glu的添加可稳定蛋白质的温度,并在展开过程中诱导早期的平衡中间产物。发现稳定性是由焓驱动的,与RNase A相比,α-LA的稳定自由能更大。在存在Glu的情况下,两种蛋白质的部分摩尔体积和可压缩性的降低表明,这些蛋白质通过表面水合达到了更紧密的状态,这可以提供更稳定的构象。分子动力学模拟研究也证明了这一点,该研究表明,添加Glu后,蛋白质周围的水密度会增加。此外,中间体可能会因较低浓度(约0.5 M)的氯化胍和盐而完全不稳定。但是,尿素会破坏由α-LA形成的Glu诱导的中间产物,而在RNase A的情况下,尿素只会稍微失稳,而RNase A具有正的表面电荷,并可能与Glu发生电荷-电荷相互作用。这表明,除了水合作用外,哥伦比亚的相互作用也可能有助于中间体的稳定性。在不存在和存在Glu的情况下,在不同温度下进行Gdm诱导的RNase A和α-LA的变性。这些结果还显示了Glu诱导的两种蛋白质的稳定。然而,在化学变性过程中,所有展开的转变都遵循两个状态的转变。对于较高温度的RNase A,Glu发挥的稳定性程度较高,而对于较低温度的α-LA,它提供的稳定性更高。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。在不存在和存在Glu的情况下,在不同温度下进行Gdm诱导的RNase A和α-LA的变性。这些结果还显示了Glu诱导的两种蛋白质的稳定。然而,在化学变性过程中,所有展开的转变都遵循两个状态的转变。对于较高温度的RNase A,Glu发挥的稳定性程度较高,而对于较低温度的α-LA,它提供的稳定性更高。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。在不存在和存在Glu的情况下,在不同温度下进行Gdm诱导的RNase A和α-LA的变性。这些结果还显示了Glu诱导的两种蛋白质的稳定。然而,在化学变性过程中,所有展开的转变都遵循两个状态的转变。对于较高温度的RNase A,Glu发挥的稳定性程度较高,而对于较低温度的α-LA,它提供的稳定性更高。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。这些结果还显示了Glu诱导的两种蛋白质的稳定。然而,在化学变性过程中,所有展开的转变都遵循两个状态的转变。对于较高温度的RNase A,Glu发挥的稳定性程度较高,而对于较低温度的α-LA,它提供的稳定性更高。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。这些结果还显示了Glu诱导的两种蛋白质的稳定。然而,在化学变性过程中,所有展开的转变都遵循两个状态的转变。对于较高温度的RNase A,Glu发挥的稳定性程度较高,而对于较低温度的α-LA,它提供的稳定性更高。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。相反,它在较低温度下为α-LA提供了更高的稳定性。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。相反,它在较低温度下为α-LA提供了更高的稳定性。因此,实验表明,Glu诱导了一个热平衡中间产物,并增加了蛋白质的热力学稳定性,而与它们的表面电荷无关。蛋白质之间的稳定性程度以温度依赖性方式变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号