当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Convenient, functional group-tolerant, transition metal-free synthesis of aryl and heteroaryl trifluoromethyl ketones with the use of methyl trifluoroacetate†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7ob02862h Kazumasa Funabiki 1, 2, 3, 4 , Ayaka Hayakawa 1, 2, 3, 4 , Toshiyasu Inuzuka 2, 3, 4, 5, 6
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7ob02862h Kazumasa Funabiki 1, 2, 3, 4 , Ayaka Hayakawa 1, 2, 3, 4 , Toshiyasu Inuzuka 2, 3, 4, 5, 6
Affiliation
|
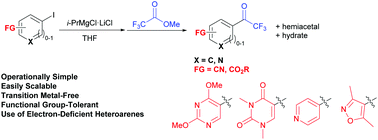
A new convenient, functional group-tolerant, transition metal-free route to aryl trifluoromethyl ketones under mild conditions is described. The reaction of not only aryl Grignard reagents carrying reducible electrophilic functional groups, such as ester and cyano groups, but also electron-deficient nitrogen-containing heteroaryl Grignard reagents with methyl trifluoroacetate gives the corresponding aryl or heteroaryl trifluoromethyl ketones in good yields. Biological assays revealed that new heteroaryl trifluoromethyl ketones carrying 2,4-dimethoxypyrimidine and 3,5-dimethylisoxazole rings show good anti-tumor activities.
中文翻译:

使用三氟乙酸甲酯方便,无官能团的,无过渡金属的芳基和杂芳基三氟甲基酮的合成†
描述了在温和条件下向芳基三氟甲基酮的一种方便的,新的,耐官能团的,无过渡金属的途径。不仅携带有可还原的亲电官能团(例如酯和氰基)的芳基格氏试剂,而且是缺电子的含氮杂芳基格氏试剂与三氟乙酸甲酯的反应,都能以良好的收率得到相应的芳基或杂芳基三氟甲基酮。生物学测定表明,带有2,4-二甲氧基嘧啶和3,5-二甲基异恶唑环的新杂芳基三氟甲基酮显示出良好的抗肿瘤活性。
更新日期:2017-12-22
中文翻译:

使用三氟乙酸甲酯方便,无官能团的,无过渡金属的芳基和杂芳基三氟甲基酮的合成†
描述了在温和条件下向芳基三氟甲基酮的一种方便的,新的,耐官能团的,无过渡金属的途径。不仅携带有可还原的亲电官能团(例如酯和氰基)的芳基格氏试剂,而且是缺电子的含氮杂芳基格氏试剂与三氟乙酸甲酯的反应,都能以良好的收率得到相应的芳基或杂芳基三氟甲基酮。生物学测定表明,带有2,4-二甲氧基嘧啶和3,5-二甲基异恶唑环的新杂芳基三氟甲基酮显示出良好的抗肿瘤活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号