当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formal total synthesis of salvianolic acid N†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7ob03025h Kong Wu 1, 2, 3, 4 , Zhong Pao Xie 1, 2, 3, 4 , Dong-Mei Cui 1, 2, 3, 4 , Chen Zhang 4, 5, 6, 7
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7ob03025h Kong Wu 1, 2, 3, 4 , Zhong Pao Xie 1, 2, 3, 4 , Dong-Mei Cui 1, 2, 3, 4 , Chen Zhang 4, 5, 6, 7
Affiliation
|
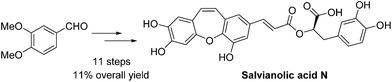
An efficient synthetic pathway for the total synthesis of salvianolic acid N has been reported. The key reaction steps, the Wittig reaction for Z-stereoselectivity and an intramolecular cyclization for a seven membered ring skeleton, have been optimized to improve the synthetic feasibility and provide the best conditions in terms of yield. Moreover, a notable reaction is the reaction of the deprotected allylic group with Pd catalyst. An improved overall yield of 11% has been achieved for salvianolic acid N starting from 3,4-dimethoxybenzaldehyde in 11 steps.
中文翻译:

的丹酸N-正式全合成†
已经报道了丹酚酸N全合成的有效合成途径。已经优化了关键反应步骤,即Z-立体选择性的Wittig反应和七元环骨架的分子内环化反应,以提高合成的可行性并提供最佳的收率条件。此外,值得注意的反应是脱保护的烯丙基与Pd催化剂的反应。从3,4-二甲氧基苯甲醛开始,分11步获得丹酚酸N的总收率提高了11%。
更新日期:2017-12-22
中文翻译:

的丹酸N-正式全合成†
已经报道了丹酚酸N全合成的有效合成途径。已经优化了关键反应步骤,即Z-立体选择性的Wittig反应和七元环骨架的分子内环化反应,以提高合成的可行性并提供最佳的收率条件。此外,值得注意的反应是脱保护的烯丙基与Pd催化剂的反应。从3,4-二甲氧基苯甲醛开始,分11步获得丹酚酸N的总收率提高了11%。









































 京公网安备 11010802027423号
京公网安备 11010802027423号