当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Closing the gap for efficient immobilization of biocatalysts in continuous processes: HaloTag™ fusion enzymes for a continuous enzymatic cascade towards a vicinal chiral diol
Green Chemistry ( IF 9.3 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7gc03225k J. Döbber 1, 2, 3, 4 , T. Gerlach 1, 2, 3, 4 , H. Offermann 1, 2, 3, 4 , D. Rother 1, 2, 3, 4 , M. Pohl 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7gc03225k J. Döbber 1, 2, 3, 4 , T. Gerlach 1, 2, 3, 4 , H. Offermann 1, 2, 3, 4 , D. Rother 1, 2, 3, 4 , M. Pohl 1, 2, 3, 4
Affiliation
|
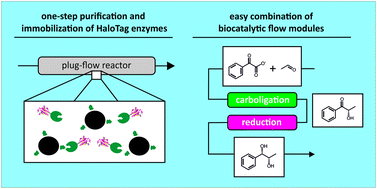
Compartmentalization of biocatalysts is an effective tool to integrate biocatalytic steps in continuous (chemo)enzymatic cascades. Therefore, efficient covalent immobilization techniques are of utmost importance, which enable a fast and selective immobilization of the enzyme directly from crude cell extracts. Here we demonstrate that the HaloTag™ mediates the covalent immobilization of such fusion enzymes in only a few minutes contact time with the respective modified carrier in a packed-bed reactor, thereby enabling enzyme immobilization directly in the flow setup. In this study, we evaluated this concept for a continuous enzymatic cascade towards a chiral vicinal diol by combining a variant of the benzoylformate decarboxylase from Pseudomonas putida (PpBFD) and the alcohol dehydrogenase from Lactobacillus brevis (LbADH). Limitations in PpBFD stability were overcome by optimization of buffer salt, cofactor concentration and choice of a different substrate. For optimal LbADH activity, excess acetaldehyde was removed in-line. This optimization lead to a high operational stability of the individual cascade steps up to several weeks and resulting in the efficient stereoselective production of (1S,2S)-1-phenylpropane-1,2-diol with high conversion up to 99%, high stereoselectivities (ee/ic) up to 96% and space–time yields up to 1850 g L−1 d−1.
中文翻译:

缩小在连续过程中有效固定生物催化剂的差距:HaloTag™融合酶可实现向邻位手性二醇的连续酶促级联反应
生物催化剂的区室化是将生物催化步骤整合到连续(化学)酶级联反应中的有效工具。因此,有效的共价固定化技术至关重要,它可以直接从粗细胞提取物中快速选择性地固定化酶。在这里,我们证明HaloTag™在填充床反应器中与相应的修饰载体仅在几分钟的接触时间内即可介导此类融合酶的共价固定化,从而使酶可以直接在流动装置中固定化。在这项研究中,我们通过结合恶臭假单胞菌(Ppudomonas putida(PpBFD)和短乳杆菌(Lb ADH)的醇脱氢酶。通过优化缓冲盐,辅因子浓度和选择其他底物,克服了Pp BFD稳定性的局限性。为了获得最佳的Lb ADH活性,在线去除了过量的乙醛。这种优化导致各个级联步骤的最高操作稳定性达到数周之久,并导致高效地立体选择性生产(1 S,2 S)-1-苯基丙烷-1,2-二醇,且转化率高达99%,高达96%的高立体选择性(ee / ic),时空产率高达1850 g L -1 d -1。
更新日期:2018-01-22
中文翻译:

缩小在连续过程中有效固定生物催化剂的差距:HaloTag™融合酶可实现向邻位手性二醇的连续酶促级联反应
生物催化剂的区室化是将生物催化步骤整合到连续(化学)酶级联反应中的有效工具。因此,有效的共价固定化技术至关重要,它可以直接从粗细胞提取物中快速选择性地固定化酶。在这里,我们证明HaloTag™在填充床反应器中与相应的修饰载体仅在几分钟的接触时间内即可介导此类融合酶的共价固定化,从而使酶可以直接在流动装置中固定化。在这项研究中,我们通过结合恶臭假单胞菌(Ppudomonas putida(PpBFD)和短乳杆菌(Lb ADH)的醇脱氢酶。通过优化缓冲盐,辅因子浓度和选择其他底物,克服了Pp BFD稳定性的局限性。为了获得最佳的Lb ADH活性,在线去除了过量的乙醛。这种优化导致各个级联步骤的最高操作稳定性达到数周之久,并导致高效地立体选择性生产(1 S,2 S)-1-苯基丙烷-1,2-二醇,且转化率高达99%,高达96%的高立体选择性(ee / ic),时空产率高达1850 g L -1 d -1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号