当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, spectroscopic investigation, and DFT study of N,N′-disubstituted ferrocene-based thiourea complexes as potent anticancer agents†
Dalton Transactions ( IF 3.5 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7dt04090c Faiza Asghar 1, 1, 2, 3, 4 , Saira Fatima 1, 4, 5, 6 , Sadaf Rana 1, 4, 5, 6 , Amin Badshah 1, 4, 5, 6 , Ian S. Butler 1, 7, 8, 9 , Muhammad Nawaz Tahir 4, 10, 11, 12
Dalton Transactions ( IF 3.5 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c7dt04090c Faiza Asghar 1, 1, 2, 3, 4 , Saira Fatima 1, 4, 5, 6 , Sadaf Rana 1, 4, 5, 6 , Amin Badshah 1, 4, 5, 6 , Ian S. Butler 1, 7, 8, 9 , Muhammad Nawaz Tahir 4, 10, 11, 12
Affiliation
|
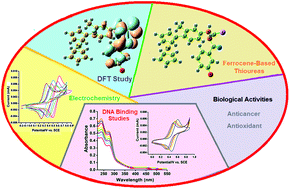
In the present work, the synthesis, characterization (FT-IR, multinuclear (1H and 13C) NMR, AAS, Raman, and elemental analyses), DNA binding (cyclic voltammetry, UV-Vis spectroscopy), and in vitro biological screening of nine new ferrocene-incorporated thioureas (A1–A9) are reported. Furthermore, the single-crystal X-ray structure of compound A8 was also determined. The ferrocene-based N,N′-disubstituted thioureas were derived by allowing the ferrocenyl anilines to react with freshly prepared isothiocyanates under a N2 atmosphere in dry acetone. The DNA binding studies performed by cyclic voltammetry and UV-Vis spectroscopy produced results that are in close agreement with one another for the binding constants (K) and an electrostatic mode of interaction was observed. The DFT/B3LYP method was used to determine the charge distribution and HOMO/LUMO energies of the optimized structure. The DFT calculated HOMO and LUMO energies correlate well with the experimentally determined redox potential values. The synthesized ferrocenyl thioureas exhibited good scavenging activity against the 1,1-diphenyl-2-picrylhydrazyl radical (DPPH). These complexes were also scanned for their in vitro cytotoxic activity against MCF-7 carcinoma cells, and also towards the non-cancerous cell line MCF-10A. The results showed modest cytotoxicity against the subjected cancer cell line compared with a standard chemotherapeutic drug (cisplatin). However, these ferrocenyl derivatives have fewer toxic effects in normal cells.
中文翻译:

N,N'-二取代的二茂铁基硫脲复合物作为强效抗癌药的 合成,光谱研究和DFT研究†
在本工作中,合成,表征(FT-IR,多核(1 H和13 C)NMR,AAS,拉曼和元素分析),DNA结合(循环伏安法,UV-Vis光谱)和体外生物学筛选据报道有九种新的二茂铁结合硫脲(A1-A9)。此外,还确定了化合物A8的单晶X射线结构。通过使二茂铁基苯胺在N 2下与新鲜制备的异硫氰酸酯反应,得到二茂铁基的N,N'-二取代的硫脲。干燥丙酮中的气氛。通过循环伏安法和UV-Vis光谱学进行的DNA结合研究得出的结果,其结合常数(K)彼此非常一致,并且观察到了相互作用的静电模式。DFT / B3LYP方法用于确定优化结构的电荷分布和HOMO / LUMO能量。DFT计算的HOMO和LUMO能量与实验确定的氧化还原电位值具有很好的相关性。合成的二茂铁基硫脲显示出对1,1-二苯基-2-吡啶并肼基(DPPH)的良好清除活性。还对这些复合物进行了体外扫描对MCF-7癌细胞以及对非癌细胞系MCF-10A具有细胞毒活性。结果显示,与标准化疗药物(顺铂)相比,对受治疗的癌细胞系具有中等程度的细胞毒性。然而,这些二茂铁基衍生物在正常细胞中具有较少的毒性作用。
更新日期:2017-12-22
中文翻译:

N,N'-二取代的二茂铁基硫脲复合物作为强效抗癌药的 合成,光谱研究和DFT研究†
在本工作中,合成,表征(FT-IR,多核(1 H和13 C)NMR,AAS,拉曼和元素分析),DNA结合(循环伏安法,UV-Vis光谱)和体外生物学筛选据报道有九种新的二茂铁结合硫脲(A1-A9)。此外,还确定了化合物A8的单晶X射线结构。通过使二茂铁基苯胺在N 2下与新鲜制备的异硫氰酸酯反应,得到二茂铁基的N,N'-二取代的硫脲。干燥丙酮中的气氛。通过循环伏安法和UV-Vis光谱学进行的DNA结合研究得出的结果,其结合常数(K)彼此非常一致,并且观察到了相互作用的静电模式。DFT / B3LYP方法用于确定优化结构的电荷分布和HOMO / LUMO能量。DFT计算的HOMO和LUMO能量与实验确定的氧化还原电位值具有很好的相关性。合成的二茂铁基硫脲显示出对1,1-二苯基-2-吡啶并肼基(DPPH)的良好清除活性。还对这些复合物进行了体外扫描对MCF-7癌细胞以及对非癌细胞系MCF-10A具有细胞毒活性。结果显示,与标准化疗药物(顺铂)相比,对受治疗的癌细胞系具有中等程度的细胞毒性。然而,这些二茂铁基衍生物在正常细胞中具有较少的毒性作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号