当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral sulfoxides: advances in asymmetric synthesis and problems with the accurate determination of the stereochemical outcome
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c6cs00703a Jianlin Han 1, 2, 3, 4, 5 , Vadim A. Soloshonok 6, 7, 8, 9, 10 , Karel D. Klika 11, 12, 13, 14 , Józef Drabowicz 15, 16, 17, 18, 19 , Alicja Wzorek 6, 7, 8, 9, 10
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1039/c6cs00703a Jianlin Han 1, 2, 3, 4, 5 , Vadim A. Soloshonok 6, 7, 8, 9, 10 , Karel D. Klika 11, 12, 13, 14 , Józef Drabowicz 15, 16, 17, 18, 19 , Alicja Wzorek 6, 7, 8, 9, 10
Affiliation
|
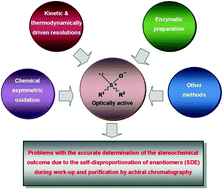
Chiral sulfoxides are in extremely high demand in nearly every sector of the chemical industry concerned with the design and development of new synthetic reagents, drugs, and functional materials. The primary objective of this review is to update readers on the latest developments from the past five years (2011–2016) in the preparation of optically active sulfoxides. Methodologies covered include catalytic asymmetric sulfoxidation using either chemical, enzymatic, or hybrid biocatalytic means; kinetic resolution involving oxidation to sulfones, reduction to sulfides, modification of side chains, and imidation to sulfoximines; as well as various other methods including nucleophilic displacement at the sulfur atom for the desymmetrization of achiral sulfoxides, enantioselective recognition and separation based on either metal–organic frameworks (MOF's) or host–guest chemistry, and the Horner–Wadsworth–Emmons reaction. A second goal of this work concerns a critical discussion of the problem of the accurate determination of the stereochemical outcome of a reaction due to the self-disproportionation of enantiomers (SDE) phenomenon, particularly as it relates to chiral sulfoxides. The SDE is a little-appreciated phenomenon that can readily and spontaneously occur for scalemic samples when subjected to practically any physicochemical process. It has now been unequivocally demonstrated that ignorance in the SDE phenomenon inevitably leads to erroneous interpretation of the stereochemical outcome of catalytic enantioselective reactions, in particular, for the synthesis of chiral sulfoxides. It is hoped that this two-pronged approach to covering the chemistry of chiral sulfoxides will be appealing, engaging, and motivating for current research-active authors to respond to in their future publications in this exciting area of current research.
中文翻译:

手性亚砜:不对称合成的进展和立体化学结果的准确测定存在问题
在涉及新合成试剂,药物和功能材料的设计和开发的化学工业中,几乎每个行业都对手性亚砜的需求量非常大。这篇综述的主要目的是向读者介绍过去五年(2011-2016年)在制备光学活性亚砜方面的最新进展。涵盖的方法包括使用化学,酶促或混合生物催化方式的催化不对称硫氧化;动力学拆分,包括氧化为砜,还原为硫化物,修饰侧链和酰亚胺化为亚磺酰亚胺;以及其他各种方法,包括用于非手性亚砜去对称化的硫原子上的亲核取代,基于金属-有机骨架(MOF's)或宿主-客体化学,以及霍纳-沃兹沃思-埃蒙斯反应的对映选择性识别和分离。这项工作的第二个目标涉及对由于对映异构体(SDE)现象的自歧化(SDE)现象,特别是与手性亚砜有关的反应的立体化学结果的准确测定问题的批判性讨论。SDE是一种鲜为人知的现象,当经历几乎任何物理化学过程时,对于鳞状样品而言,它很容易自发地发生。现已明确表明,SDE现象的无知不可避免地导致对催化对映选择性反应的立体化学结果的错误解释,特别是对于手性亚砜的合成。
更新日期:2017-12-22
中文翻译:

手性亚砜:不对称合成的进展和立体化学结果的准确测定存在问题
在涉及新合成试剂,药物和功能材料的设计和开发的化学工业中,几乎每个行业都对手性亚砜的需求量非常大。这篇综述的主要目的是向读者介绍过去五年(2011-2016年)在制备光学活性亚砜方面的最新进展。涵盖的方法包括使用化学,酶促或混合生物催化方式的催化不对称硫氧化;动力学拆分,包括氧化为砜,还原为硫化物,修饰侧链和酰亚胺化为亚磺酰亚胺;以及其他各种方法,包括用于非手性亚砜去对称化的硫原子上的亲核取代,基于金属-有机骨架(MOF's)或宿主-客体化学,以及霍纳-沃兹沃思-埃蒙斯反应的对映选择性识别和分离。这项工作的第二个目标涉及对由于对映异构体(SDE)现象的自歧化(SDE)现象,特别是与手性亚砜有关的反应的立体化学结果的准确测定问题的批判性讨论。SDE是一种鲜为人知的现象,当经历几乎任何物理化学过程时,对于鳞状样品而言,它很容易自发地发生。现已明确表明,SDE现象的无知不可避免地导致对催化对映选择性反应的立体化学结果的错误解释,特别是对于手性亚砜的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号