当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Studies on the Michael Addition of Amines and Hydrazines To Nitrostyrenes: Nitroalkane Elimination via a Retro-aza-Henry-Type Process
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.joc.7b02637 Michael G. Kallitsakis 1 , Peter D. Tancini 2 , Mudit Dixit 2 , Giannis Mpourmpakis 2 , Ioannis N. Lykakis 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.joc.7b02637 Michael G. Kallitsakis 1 , Peter D. Tancini 2 , Mudit Dixit 2 , Giannis Mpourmpakis 2 , Ioannis N. Lykakis 1
Affiliation
|
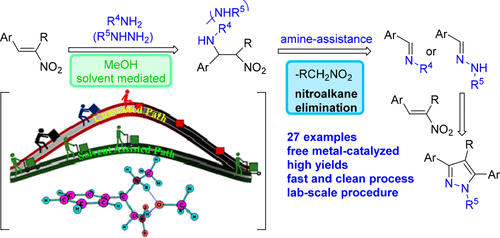
In this article we report on the mechanistic studies of the Michael addition of amines and hydrazines to nitrostyrenes. Under the present conditions, the corresponding N-alkyl/aryl substituted benzyl imines and N-methyl/phenyl substituted benzyl hydrazones were observed via a retro-aza-Henry-type process. By combining organic synthesis and characterization experiments with computational chemistry calculations, we reveal that this reaction proceeds via a protic solvent-mediated mechanism. Experiments in deuterated methanol CD3OD reveal the synthesis and isolation of the corresponding deuterated intermediated Michael adduct, results that support the proposed slovent-mediated pathway. From the synthetic point of view, the reaction occurs under mild, noncatalytic conditions and can be used as a useful platform to yield the biologically important N-methyl pyrazoles in a one-pot manner, simple starting with the corresponding nitrostyrenes and the methylhydrazine.
中文翻译:

胺和肼向硝基苯乙烯的迈克尔加成反应的机理研究:通过逆转氮杂亨利型方法消除硝基烷
在本文中,我们报告了将胺和肼加成至硝基苯乙烯的机理研究。在当前条件下,通过逆转氮杂亨利型方法观察到了相应的N-烷基/芳基取代的苄基亚胺和N-甲基/苯基取代的苄基hydr。通过将有机合成和表征实验与计算化学计算相结合,我们揭示了该反应是通过质子溶剂介导的机理进行的。氘代甲醇CD 3的实验OD揭示了相应的氘代中间体迈克尔加合物的合成和分离,结果支持所提出的slovent介导的途径。从合成的观点来看,该反应在温和的非催化条件下进行,可以用作一锅法以生物学上重要的N-甲基吡唑的有用平台,简单地从相应的硝基苯乙烯和甲基肼开始。
更新日期:2018-01-11
中文翻译:

胺和肼向硝基苯乙烯的迈克尔加成反应的机理研究:通过逆转氮杂亨利型方法消除硝基烷
在本文中,我们报告了将胺和肼加成至硝基苯乙烯的机理研究。在当前条件下,通过逆转氮杂亨利型方法观察到了相应的N-烷基/芳基取代的苄基亚胺和N-甲基/苯基取代的苄基hydr。通过将有机合成和表征实验与计算化学计算相结合,我们揭示了该反应是通过质子溶剂介导的机理进行的。氘代甲醇CD 3的实验OD揭示了相应的氘代中间体迈克尔加合物的合成和分离,结果支持所提出的slovent介导的途径。从合成的观点来看,该反应在温和的非催化条件下进行,可以用作一锅法以生物学上重要的N-甲基吡唑的有用平台,简单地从相应的硝基苯乙烯和甲基肼开始。











































 京公网安备 11010802027423号
京公网安备 11010802027423号