Synthesis ( IF 2.2 ) Pub Date : 2017-12-21 , DOI: 10.1055/s-0036-1591745 Marcus Baumann 1, 2 , Ian Baxendale 1
|
Abstract
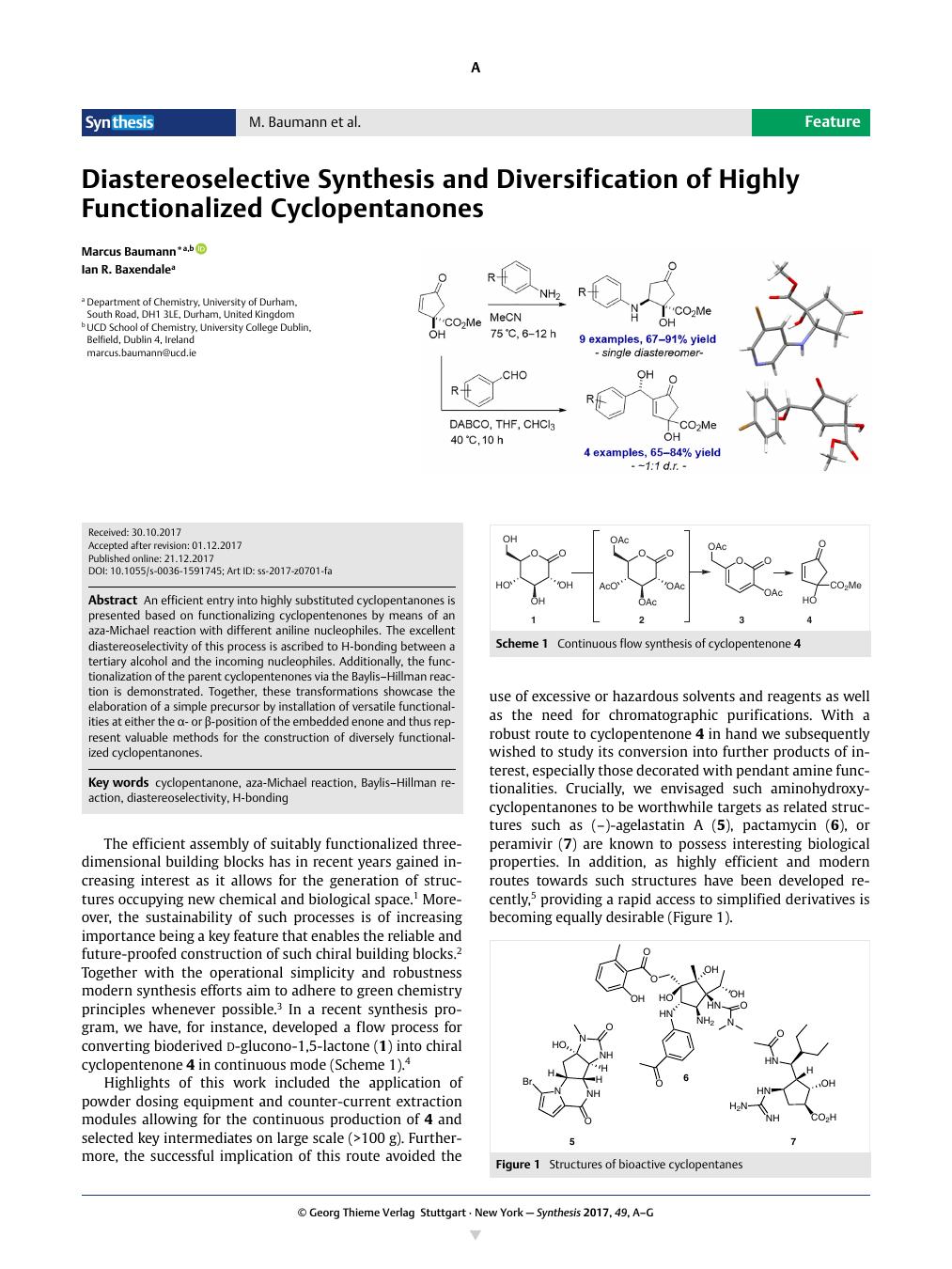
An efficient entry into highly substituted cyclopentanones is presented based on functionalizing cyclopentenones by means of an aza-Michael reaction with different aniline nucleophiles. The excellent diastereoselectivity of this process is ascribed to H-bonding between a tertiary alcohol and the incoming nucleophiles. Additionally, the functionalization of the parent cyclopentenones via the Baylis–Hillman reaction is demonstrated. Together, these transformations showcase the elaboration of a simple precursor by installation of versatile functionalities at either the α- or β-position of the embedded enone and thus represent valuable methods for the construction of diversely functionalized cyclopentanones.
An efficient entry into highly substituted cyclopentanones is presented based on functionalizing cyclopentenones by means of an aza-Michael reaction with different aniline nucleophiles. The excellent diastereoselectivity of this process is ascribed to H-bonding between a tertiary alcohol and the incoming nucleophiles. Additionally, the functionalization of the parent cyclopentenones via the Baylis–Hillman reaction is demonstrated. Together, these transformations showcase the elaboration of a simple precursor by installation of versatile functionalities at either the α- or β-position of the embedded enone and thus represent valuable methods for the construction of diversely functionalized cyclopentanones.
中文翻译:

高功能化环戊酮的非对映选择性合成和多样化
摘要
基于通过与不同的苯胺亲核试剂的氮杂-迈克尔反应来官能化环戊烯酮,提出了高效进入高度取代的环戊烯酮的方法。该过程的极好的非对映选择性是由于叔醇与进入的亲核试剂之间的氢键作用。另外,通过Baylis-Hillman反应证明了母体环戊烯酮的功能化。总之,这些转变展示了通过在嵌入的烯酮的α-或β-位上安装通用功能来制备简单的前体的过程,因此代表了构建各种功能化的环戊酮的有价值的方法。
基于通过与不同的苯胺亲核试剂的氮杂-迈克尔反应来官能化环戊烯酮,提出了高效进入高度取代的环戊烯酮的方法。该过程的极好的非对映选择性是由于叔醇与进入的亲核试剂之间的氢键作用。另外,通过Baylis-Hillman反应证明了母体环戊烯酮的功能化。总之,这些转变展示了通过在嵌入的烯酮的α-或β-位上安装通用功能来制备简单的前体的过程,因此代表了构建各种功能化的环戊酮的有价值的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号