当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Counterion-Release Entropy Governs the Inhibition of Serum Proteins by Polyelectrolyte Drugs
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.biomac.7b01499 Xiao Xu 1, 2 , Qidi Ran 1, 3, 4 , Pradip Dey 4, 5 , Rohit Nikam 1, 2 , Rainer Haag 3, 4 , Matthias Ballauff 1, 2, 3 , Joachim Dzubiella 1, 2, 3
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.biomac.7b01499 Xiao Xu 1, 2 , Qidi Ran 1, 3, 4 , Pradip Dey 4, 5 , Rohit Nikam 1, 2 , Rainer Haag 3, 4 , Matthias Ballauff 1, 2, 3 , Joachim Dzubiella 1, 2, 3
Affiliation
|
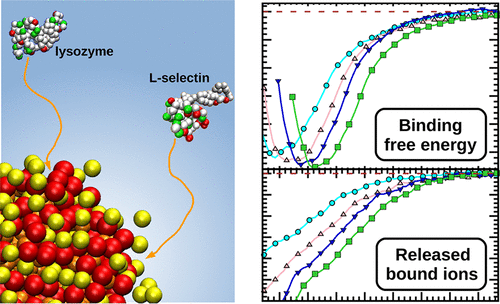
Dendritic polyelectrolytes constitute high potential drugs and carrier systems for biomedical purposes. Still, their biomolecular interaction modes, in particular those determining the binding affinity to proteins, have not been rationalized. We study the interaction of the drug candidate dendritic polyglycerol sulfate (dPGS) with serum proteins using isothermal titration calorimetry (ITC) interpreted and complemented with molecular computer simulations. Lysozyme is first studied as a well-defined model protein to verify theoretical concepts, which are then applied to the important cell adhesion protein family of selectins. We demonstrate that the driving force of the strong complexation, leading to a distinct protein corona, originates mainly from the release of only a few condensed counterions from the dPGS upon binding. The binding constant shows a surprisingly weak dependence on dPGS size (and bare charge) which can be understood by colloidal charge-renormalization effects and by the fact that the magnitude of the dominating counterion-release mechanism almost exclusively depends on the interfacial charge structure of the protein-specific binding patch. Our findings explain the high selectivity of P- and L-selectins over E-selectin for dPGS to act as a highly anti-inflammatory drug. The entire analysis demonstrates that the interaction of proteins with charged polymeric drugs can be predicted by simulations with unprecedented accuracy. Thus, our results open new perspectives for the rational design of charged polymeric drugs and carrier systems.
中文翻译:

抗衡离子释放熵决定了聚电解质药物对血清蛋白的抑制作用
树突状聚电解质构成了用于生物医学目的的高潜力药物和载体系统。仍然,它们的生物分子相互作用模式,特别是那些确定对蛋白质的结合亲和力的模式尚未得到合理化。我们使用等温滴定量热法(ITC)研究了候选药物树突状聚甘油硫酸盐(dPGS)与血清蛋白的相互作用,并用分子计算机模拟进行了补充。首先将溶菌酶作为一种定义明确的模型蛋白进行研究,以验证理论概念,然后将其应用于重要的选择素细胞粘附蛋白家族。我们证明了强络合的驱动力,导致独特的蛋白质电晕,主要来源于结合后仅从dPGS释放出少数缩合抗衡离子。结合常数显示出对dPGS大小(和裸电荷)的出乎意料的弱依赖性,这可以通过胶体电荷重新归一化作用以及主要的抗衡离子释放机制的强度几乎完全取决于DPGS的界面电荷结构的事实来理解。蛋白质特异性结合补丁。我们的发现解释了P-和L-选择素相对于E-选择素对dPGS充当高度抗炎药的高度选择性。整个分析表明,可以通过模拟以前所未有的准确性预测蛋白质与带电聚合物药物的相互作用。因此,我们的结果为带电聚合物药物和载体系统的合理设计开辟了新的前景。
更新日期:2018-01-10
中文翻译:

抗衡离子释放熵决定了聚电解质药物对血清蛋白的抑制作用
树突状聚电解质构成了用于生物医学目的的高潜力药物和载体系统。仍然,它们的生物分子相互作用模式,特别是那些确定对蛋白质的结合亲和力的模式尚未得到合理化。我们使用等温滴定量热法(ITC)研究了候选药物树突状聚甘油硫酸盐(dPGS)与血清蛋白的相互作用,并用分子计算机模拟进行了补充。首先将溶菌酶作为一种定义明确的模型蛋白进行研究,以验证理论概念,然后将其应用于重要的选择素细胞粘附蛋白家族。我们证明了强络合的驱动力,导致独特的蛋白质电晕,主要来源于结合后仅从dPGS释放出少数缩合抗衡离子。结合常数显示出对dPGS大小(和裸电荷)的出乎意料的弱依赖性,这可以通过胶体电荷重新归一化作用以及主要的抗衡离子释放机制的强度几乎完全取决于DPGS的界面电荷结构的事实来理解。蛋白质特异性结合补丁。我们的发现解释了P-和L-选择素相对于E-选择素对dPGS充当高度抗炎药的高度选择性。整个分析表明,可以通过模拟以前所未有的准确性预测蛋白质与带电聚合物药物的相互作用。因此,我们的结果为带电聚合物药物和载体系统的合理设计开辟了新的前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号