当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Activity of an Iron-Based Water Oxidation Catalyst: Substrate Effects of Graphitic Electrodes.
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-12-21 , DOI: 10.1021/acscatal.7b03284 Konstantin G Kottrup 1 , Silvia D'Agostini 1 , Phebe H van Langevelde 1 , Maxime A Siegler 2 , Dennis G H Hetterscheid 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2017-12-21 , DOI: 10.1021/acscatal.7b03284 Konstantin G Kottrup 1 , Silvia D'Agostini 1 , Phebe H van Langevelde 1 , Maxime A Siegler 2 , Dennis G H Hetterscheid 1
Affiliation
|
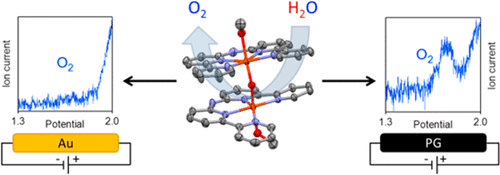
The synthesis, characterization, and electrochemical studies of the dinuclear complex [(MeOH)Fe(Hbbpya)-μ-O-(Hbbpya)Fe(MeOH)](OTf)4 (1) (with Hbbpya = N,N-bis(2,2'-bipyrid-6-yl)amine) are described. With the help of online electrochemical mass spectrometry, the complex is demonstrated to be active as a water oxidation catalyst. Comparing the results obtained for different electrode materials shows a clear substrate influence of the electrode, as the complex shows a significantly lower catalytic overpotential on graphitic working electrodes in comparison to other electrode materials. Cyclic voltammetry experiments provide evidence that the structure of complex 1 undergoes reversible changes under high-potential conditions, regenerating the original structure of complex 1 upon returning to lower potentials. Results from electrochemical quartz crystal microbalance experiments rule out that catalysis proceeds via deposition of catalytically active material on the electrode surface.
中文翻译:

铁基水氧化催化剂的催化活性:石墨电极的底物效应。
双核络合物[(MeOH)Fe(Hbbpya)-μ-O-(Hbbpya)Fe(MeOH)](OTf)4(1)的合成,表征和电化学研究(Hbbpya = N,N-bis(描述了2,2′-联吡啶-6-基)胺。借助于在线电化学质谱法,该络合物被证明具有水氧化催化剂的活性。比较对于不同电极材料获得的结果,显示了电极对基材的明显影响,因为与其他电极材料相比,该配合物在石墨工作电极上显示出明显更低的催化超电势。循环伏安法实验提供了证据,表明复合物1的结构在高电势条件下发生可逆变化,并在返回较低电势时再生了复合物1的原始结构。
更新日期:2018-01-10
中文翻译:

铁基水氧化催化剂的催化活性:石墨电极的底物效应。
双核络合物[(MeOH)Fe(Hbbpya)-μ-O-(Hbbpya)Fe(MeOH)](OTf)4(1)的合成,表征和电化学研究(Hbbpya = N,N-bis(描述了2,2′-联吡啶-6-基)胺。借助于在线电化学质谱法,该络合物被证明具有水氧化催化剂的活性。比较对于不同电极材料获得的结果,显示了电极对基材的明显影响,因为与其他电极材料相比,该配合物在石墨工作电极上显示出明显更低的催化超电势。循环伏安法实验提供了证据,表明复合物1的结构在高电势条件下发生可逆变化,并在返回较低电势时再生了复合物1的原始结构。







































 京公网安备 11010802027423号
京公网安备 11010802027423号