当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Beryllium Bonding in the Light of Modern Quantum Chemical Topology Tools
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jpca.7b10714 José Luis Casals-Sainz 1 , Fernando Jiménez-Grávalos 1 , Aurora Costales 1 , Evelio Francisco 1 , Ángel Martín Pendás 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-01-16 00:00:00 , DOI: 10.1021/acs.jpca.7b10714 José Luis Casals-Sainz 1 , Fernando Jiménez-Grávalos 1 , Aurora Costales 1 , Evelio Francisco 1 , Ángel Martín Pendás 1
Affiliation
|
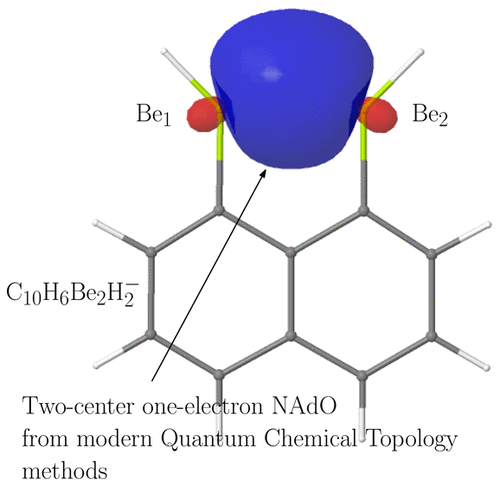
We apply several modern quantum chemical topology (QCT) tools to explore the chemical bonding in well established beryllium bonds. By using the interacting quantum atoms (IQA) approach together with electron distribution functions (EDF) and the natural adaptive orbitals (NAdOs) picture, we show that, in agreement with orbital-based analyses, the interaction in simple σ and π complexes formed by BeX2 (X = H, F, Cl) with water, ammonia, ethylene, and acetylene is dominated by electrostatic terms, albeit covalent contributions cannot be ignored. Our detailed analysis proves that several σ back-donation channels are relevant in these dimers, actually controlling the conformational preference in the π adducts. A number of one-electron beryllium bonds are also studied. Orbital invariant real space arguments clearly show that the role of covalency and charge transfer cannot be ignored.
中文翻译:

借助现代量子化学拓扑工具进行铍键合
我们应用了几种现代的量子化学拓扑(QCT)工具来探索良好建立的铍键中的化学键。通过使用相互作用的量子原子(IQA)方法以及电子分布函数(EDF)和自然自适应轨道(NAdOs)图片,我们表明,与基于轨道的分析一致,由BeX 2(X = H,F,Cl)与水,氨,乙烯和乙炔的关系主要由静电术语决定,尽管共价贡献不可忽略。我们的详细分析证明,在这些二聚体中有几个σ反向供体通道相关,实际上控制了π加合物的构象偏爱。还研究了许多单电子铍键。轨道不变实空间论证清楚地表明,共价和电荷转移的作用不容忽视。
更新日期:2018-01-16
中文翻译:

借助现代量子化学拓扑工具进行铍键合
我们应用了几种现代的量子化学拓扑(QCT)工具来探索良好建立的铍键中的化学键。通过使用相互作用的量子原子(IQA)方法以及电子分布函数(EDF)和自然自适应轨道(NAdOs)图片,我们表明,与基于轨道的分析一致,由BeX 2(X = H,F,Cl)与水,氨,乙烯和乙炔的关系主要由静电术语决定,尽管共价贡献不可忽略。我们的详细分析证明,在这些二聚体中有几个σ反向供体通道相关,实际上控制了π加合物的构象偏爱。还研究了许多单电子铍键。轨道不变实空间论证清楚地表明,共价和电荷转移的作用不容忽视。











































 京公网安备 11010802027423号
京公网安备 11010802027423号