当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of H2 by Gadolinium Cation (Gd+): Bond Energy of GdH+ and Mechanistic Insights from Guided Ion Beam and Theoretical Studies
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.jpca.7b11471 Maria Demireva 1 , P. B. Armentrout 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.jpca.7b11471 Maria Demireva 1 , P. B. Armentrout 1
Affiliation
|
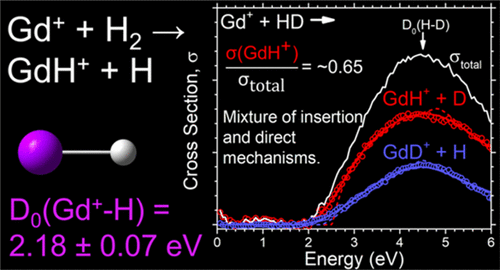
The energy-dependent reactions of the lanthanide gadolinium cation (Gd+) with H2, D2, and HD are investigated using guided ion beam tandem mass spectrometry. From analysis of the resulting endothermic product ion cross sections, the 0 K bond dissociation energy for GdH+ is measured to be 2.18 ± 0.07 eV. Theoretical calculations on GdH+ are performed for comparison with the experimental thermochemistry and generally appear to overestimate the experimental GdH+ bond dissociation energy. The branching ratio of the products in the HD reaction suggests that Gd+ reacts primarily via a statistical insertion mechanism to form the hydride product ion with contributions from direct mechanisms. Relaxed potential energy surfaces for GdH2+ are computed and are consistent with the availability of both statistical and direct reaction pathways. The reactivity and hydride bond energy for Gd+ is compared with previous results for the group three metal cations, Sc+ and Y+, and the lanthanides, La+ and Lu+, and periodic trends are discussed.
中文翻译:

Ga阳离子(Gd +)活化H 2:GdH +的键能以及离子束和理论研究的机理性见解
使用引导离子束串联质谱法研究了镧系g阳离子(Gd +)与H 2,D 2和HD的能量依赖反应。通过分析所得的吸热产物离子截面,测得GdH +的0 K键解离能为2.18±0.07 eV。对GdH +进行理论计算是为了与实验热化学进行比较,并且通常似乎高估了实验GdH +键的解离能。HD反应中产物的支化比表明Gd +反应主要是通过统计插入机制发生的,在直接机制的作用下形成了氢化物产物离子。计算出GdH 2 +的弛豫势能面,并与统计和直接反应路径的可用性一致。将Gd +的反应性和氢键能与之前的三个金属阳离子Sc +和Y +以及镧系元素La +和Lu +的结果进行了比较,并讨论了其周期性趋势。
更新日期:2018-01-12
中文翻译:

Ga阳离子(Gd +)活化H 2:GdH +的键能以及离子束和理论研究的机理性见解
使用引导离子束串联质谱法研究了镧系g阳离子(Gd +)与H 2,D 2和HD的能量依赖反应。通过分析所得的吸热产物离子截面,测得GdH +的0 K键解离能为2.18±0.07 eV。对GdH +进行理论计算是为了与实验热化学进行比较,并且通常似乎高估了实验GdH +键的解离能。HD反应中产物的支化比表明Gd +反应主要是通过统计插入机制发生的,在直接机制的作用下形成了氢化物产物离子。计算出GdH 2 +的弛豫势能面,并与统计和直接反应路径的可用性一致。将Gd +的反应性和氢键能与之前的三个金属阳离子Sc +和Y +以及镧系元素La +和Lu +的结果进行了比较,并讨论了其周期性趋势。











































 京公网安备 11010802027423号
京公网安备 11010802027423号