当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acid-Catalyzed Ring Opening of Furan in Aqueous Solution
Energy & Fuels ( IF 5.2 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.energyfuels.7b03239 Xiao Liang 1 , Brian S. Haynes 1 , Alejandro Montoya 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.energyfuels.7b03239 Xiao Liang 1 , Brian S. Haynes 1 , Alejandro Montoya 1
Affiliation
|
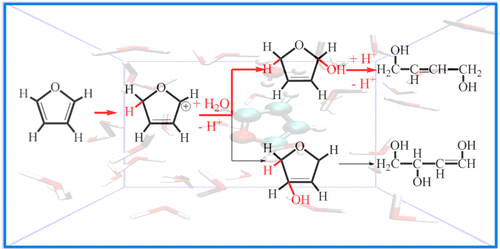
The mechanism and energetics of furan decomposition via the opening of the five-membered ring structure in dilute acid solution were investigated using density functional Car–Parrinello molecular dynamics combined with metadynamics simulations. Diffusion of an acidic proton from the aqueous medium leading to the formation of a protonated furan is found to be the rate-limiting step of the ring-opening process, with protonation at the Cα position being 7 kcal mol–1 less activated than that at the Cβ position. Protonation at the Cα position results in the formation of 2,5-dihydro-2-furanol or 2,3-dihydro-3-furanol via nucleophilic attack by a solvent molecule. Subsequent protonation of the furanols at the ring oxygen initiates the opening of the furanic ring, leading to the formation of 4-hydroxy-2-butenal.
中文翻译:

水溶液中呋喃的酸催化开环
通过使用密度泛函的Car-Parrinello分子动力学和元动力学模拟,研究了在稀酸溶液中通过五元环结构的打开导致呋喃分解的机理和能量。从导致质子化呋喃形成的水性介质中的酸性质子的扩散被发现是开环过程的速率限制步骤,用质子化在C α位置是7千卡摩尔-1小于激活在Cβ位置。质子化在C α位置导致经由溶剂分子的亲核攻击而形成2,5-二氢-2-呋喃醇或2,3-二氢-3-呋喃醇。随后在环氧处呋喃醇的质子化引发呋喃环的打开,导致形成4-羟基-2-丁烯醛。
更新日期:2017-12-20
中文翻译:

水溶液中呋喃的酸催化开环
通过使用密度泛函的Car-Parrinello分子动力学和元动力学模拟,研究了在稀酸溶液中通过五元环结构的打开导致呋喃分解的机理和能量。从导致质子化呋喃形成的水性介质中的酸性质子的扩散被发现是开环过程的速率限制步骤,用质子化在C α位置是7千卡摩尔-1小于激活在Cβ位置。质子化在C α位置导致经由溶剂分子的亲核攻击而形成2,5-二氢-2-呋喃醇或2,3-二氢-3-呋喃醇。随后在环氧处呋喃醇的质子化引发呋喃环的打开,导致形成4-羟基-2-丁烯醛。











































 京公网安备 11010802027423号
京公网安备 11010802027423号