当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Aspects of Acrylic Acid Formation from CO2–Ethylene Coupling over Palladium‐ and Nickel‐based Catalysts
ChemCatChem ( IF 3.8 ) Pub Date : 2018-02-22 , DOI: 10.1002/cctc.201701763 Yuanhui Li 1 , Zhen Liu 1 , Ruihua Cheng 1 , Boping Liu 1, 2
ChemCatChem ( IF 3.8 ) Pub Date : 2018-02-22 , DOI: 10.1002/cctc.201701763 Yuanhui Li 1 , Zhen Liu 1 , Ruihua Cheng 1 , Boping Liu 1, 2
Affiliation
|
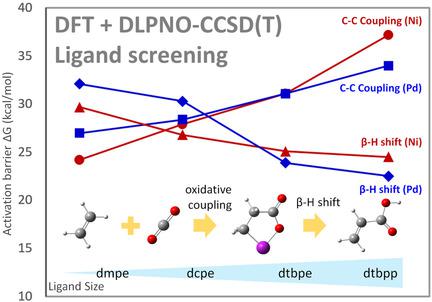
The detailed mechanism of catalytic conversion of CO2 and ethylene to acrylic acid has been investigated through DFT and DLPNO‐CCSD(T) calculations. Four bidentate ligands, dmpe (1,2‐bis(dimethylphosphino)ethane), dcpe (1,2‐bis(dicyclohexylphosphino)ethane), dtbpe (1,2‐bis(di‐tert‐butylphosphino)ethane), and tmeda (tetramethylethylenediamine) were systematically studied to understand the different catalytic behavior of the Pd‐based catalyst and the well‐known Ni‐based catalyst. The energy barrier of Pd‐/Ni‐catalyzed C−C coupling appears to increase with the larger steric hindrance of the ligand, whereas the barrier of the corresponding β‐H elimination shows an opposite tendency. The barrier for C−C coupling is likely higher than the associated barrier for β‐H elimination in both catalytic systems provided that the coordinated ligand is bulky enough. The palladium catalytic center is more effective than the nickel center for the C−C coupling and β‐H elimination steps in the presence of bulky ligands, whereas the nickel catalyst in turn performs better with small ligands. The palladium catalyst tends to be superior to the nickel catalyst during the following hydrogen transfer to the O atom. In general, the diamine ligand (tmeda) is less efficient than the diphosphine ligands for both systems. In the absence of the auxiliaries, a novel chelating carbene ligand was proposed to be quite efficient for Pd‐catalyzed C−C coupling. Two ligands (dcpm, dtbpm) showed relatively better performance towards the palladium‐catalyzed acrylic acid formation reaction among all the investigated ligands.
中文翻译:

钯和镍基催化剂上CO2-乙烯偶联形成丙烯酸的机理
通过DFT和DLPNO-CCSD(T)计算研究了CO 2和乙烯催化转化为丙烯酸的详细机理。四个双齿配体,dmpe(1,2-双(二甲基膦基)乙烷),dcpe(1,2-双(二环己基膦基)乙烷),d t bpe(1,2-双(二叔丁基)系统地研究了丁基丁基膦基乙烷和四甲基乙二胺,以了解Pd基催化剂和著名的Ni基催化剂的不同催化行为。Pd / Ni催化的C-C偶联的能垒似乎随着配体的较大位阻而增加,而相应的β-H消除的能垒却显示出相反的趋势。如果配位体足够大,则在两个催化系统中,CC偶联的障碍都可能高于相关的β-H消除障碍。在存在大量配体的情况下,钯催化中心比镍中心更有效地进行C-C偶联和β-H消除步骤,而镍催化剂则在较小的配体下表现更好。在随后的氢转移到O原子的过程中,钯催化剂倾向于优于镍催化剂。通常,对于两个系统,二胺配体(tmeda)的效率均低于二膦配体。在没有助剂的情况下,提出了一种新型的螯合卡宾配体对于Pd催化的C-C偶联非常有效。两个配体(dcpm,dt bpm)在所有研究的配体中对钯催化的丙烯酸形成反应表现出相对较好的性能。
更新日期:2018-02-22
中文翻译:

钯和镍基催化剂上CO2-乙烯偶联形成丙烯酸的机理
通过DFT和DLPNO-CCSD(T)计算研究了CO 2和乙烯催化转化为丙烯酸的详细机理。四个双齿配体,dmpe(1,2-双(二甲基膦基)乙烷),dcpe(1,2-双(二环己基膦基)乙烷),d t bpe(1,2-双(二叔丁基)系统地研究了丁基丁基膦基乙烷和四甲基乙二胺,以了解Pd基催化剂和著名的Ni基催化剂的不同催化行为。Pd / Ni催化的C-C偶联的能垒似乎随着配体的较大位阻而增加,而相应的β-H消除的能垒却显示出相反的趋势。如果配位体足够大,则在两个催化系统中,CC偶联的障碍都可能高于相关的β-H消除障碍。在存在大量配体的情况下,钯催化中心比镍中心更有效地进行C-C偶联和β-H消除步骤,而镍催化剂则在较小的配体下表现更好。在随后的氢转移到O原子的过程中,钯催化剂倾向于优于镍催化剂。通常,对于两个系统,二胺配体(tmeda)的效率均低于二膦配体。在没有助剂的情况下,提出了一种新型的螯合卡宾配体对于Pd催化的C-C偶联非常有效。两个配体(dcpm,dt bpm)在所有研究的配体中对钯催化的丙烯酸形成反应表现出相对较好的性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号