Chem ( IF 19.1 ) Pub Date : 2017-12-21 , DOI: 10.1016/j.chempr.2017.11.004 Takashi Koike , Munetaka Akita
|
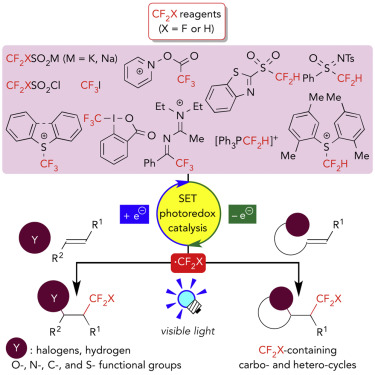
Radical tri- and difluoromethylation (fluoromethylation) by photoredox catalysis has emerged as a new synthetic methodology over the past several years. In particular, the strategy is useful for the introduction of a fluoromethyl group (CF2X, X = F or H) and a different functional group across a carbon-carbon double bond via a single operation. Step-economical synthesis of diverse organofluorine compounds bearing a C(sp3)–CF2X bond has been achieved. In this review, the discussion focuses on recent representative examples of photoredox-catalyzed fluoromethylative difunctionalization of alkenes through redox-neutral processes. Close attention is paid to the choice of the fluoromethylating reagent and the photocatalyst. The basic concept, reaction design, and the prospects for this research area are also discussed.
中文翻译:

烯烃的光催化氟甲基化双官能化的新视野
在过去的几年中,通过光氧化还原催化进行的自由基三氟甲基化和二氟甲基化(氟甲基化)已成为一种新的合成方法。特别地,该策略对于通过一次操作跨碳-碳双键引入氟甲基(CF 2 X,X = F或H)和不同的官能团是有用的。分步经济地合成各种带有C(sp 3)–CF 2的有机氟化合物X键已实现。在这篇综述中,讨论集中在通过氧化还原中性过程进行的光氧化还原催化的烯烃的氟甲基化双官能化的最新代表性实例。密切注意氟甲基化试剂和光催化剂的选择。还讨论了该研究领域的基本概念,反应设计和前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号