当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A BF3·Et2O catalyzed atom-economical approach to highly substituted indole-3-carbinols from nitrosobenzenes and propargylic alcohols†‡
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1039/c7ob02559a Sengodagounder Muthusamy 1, 2, 3, 4 , Alagesan Balasubramani 1, 2, 3, 4 , Eringathodi Suresh 4, 5, 6, 7
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1039/c7ob02559a Sengodagounder Muthusamy 1, 2, 3, 4 , Alagesan Balasubramani 1, 2, 3, 4 , Eringathodi Suresh 4, 5, 6, 7
Affiliation
|
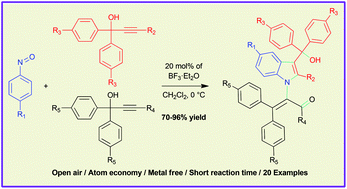
The BF3·Et2O catalyzed reaction of nitrosobenzenes and an excess amount of propargylic alcohols was investigated to synthesize highly-substituted indole-3-carbinols. This reaction involves formal [3 + 2]-cycloaddition and the subsequent 1,3-rearrangement in a tandem manner via 3-alkylidene-3H-indole N-oxides. This methodology involves the sequential addition of propargylic alcohols and inexpensive Lewis acid catalyst, occurs in an open-air environment and is atom economical. The highly-substituted indole-3-carbinols were obtained in short time and the operational simplicity allows for a large scale experiment.
中文翻译:

BF 3 ·Et 2 O催化的亚硝基苯和炔丙醇中高度取代的吲哚-3-甲醇的原子经济方法† ‡
研究了亚硝基苯与过量的炔丙醇的BF 3 ·Et 2 O催化反应,合成了高度取代的吲哚-3-甲醇。该反应包括通过3-亚烷基-3 H-吲哚N-氧化物以串联方式进行正式的[3 + 2]-环加成和随后的1,3-重排。该方法学涉及顺序添加炔丙醇和廉价的路易斯酸催化剂,发生在露天环境中并且是原子经济的。在短时间内获得了高度取代的吲哚-3-甲醇,并且操作简便允许进行大规模实验。
更新日期:2017-12-21
中文翻译:

BF 3 ·Et 2 O催化的亚硝基苯和炔丙醇中高度取代的吲哚-3-甲醇的原子经济方法† ‡
研究了亚硝基苯与过量的炔丙醇的BF 3 ·Et 2 O催化反应,合成了高度取代的吲哚-3-甲醇。该反应包括通过3-亚烷基-3 H-吲哚N-氧化物以串联方式进行正式的[3 + 2]-环加成和随后的1,3-重排。该方法学涉及顺序添加炔丙醇和廉价的路易斯酸催化剂,发生在露天环境中并且是原子经济的。在短时间内获得了高度取代的吲哚-3-甲醇,并且操作简便允许进行大规模实验。











































 京公网安备 11010802027423号
京公网安备 11010802027423号